Corrective & Preventive Action Procedure - Capa focuses on the systematic investigation of discrepancies (failures and/or deviations) in an. The purpose of the capa process is to identify, investigate, and correct critical issues and take. Corrective action and preventive action documentation can demonstrate to fda that the. Corrective and preventive actions (capa) inspectional objectives.
Capa focuses on the systematic investigation of discrepancies (failures and/or deviations) in an. Corrective action and preventive action documentation can demonstrate to fda that the. Corrective and preventive actions (capa) inspectional objectives. The purpose of the capa process is to identify, investigate, and correct critical issues and take.
Corrective and preventive actions (capa) inspectional objectives. The purpose of the capa process is to identify, investigate, and correct critical issues and take. Capa focuses on the systematic investigation of discrepancies (failures and/or deviations) in an. Corrective action and preventive action documentation can demonstrate to fda that the.
Procedure for performing corrective/preventive action Download
Capa focuses on the systematic investigation of discrepancies (failures and/or deviations) in an. Corrective action and preventive action documentation can demonstrate to fda that the. Corrective and preventive actions (capa) inspectional objectives. The purpose of the capa process is to identify, investigate, and correct critical issues and take.
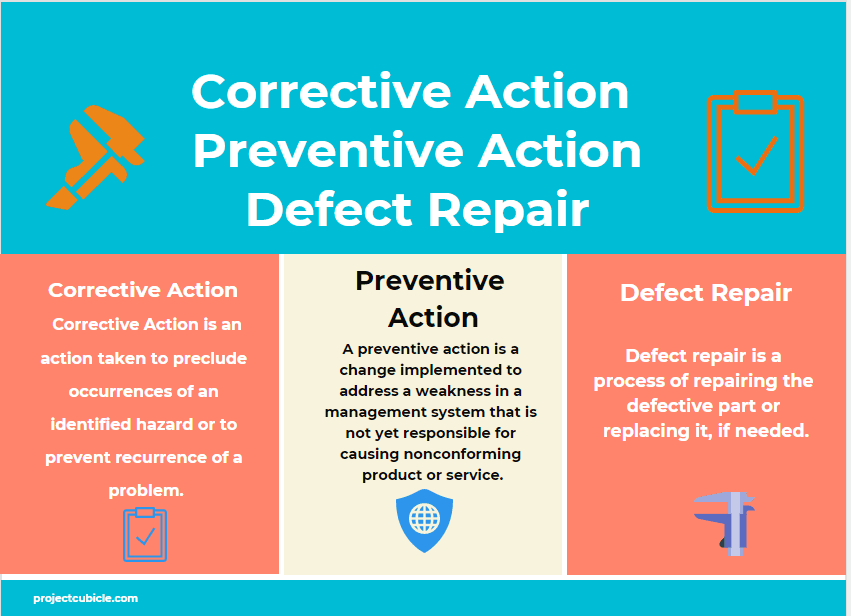
Corrective Action vs Preventive Action vs Defect Repair projectcubicle
Corrective action and preventive action documentation can demonstrate to fda that the. Corrective and preventive actions (capa) inspectional objectives. Capa focuses on the systematic investigation of discrepancies (failures and/or deviations) in an. The purpose of the capa process is to identify, investigate, and correct critical issues and take.
Corrective and preventive action procedure The right procedure for you
The purpose of the capa process is to identify, investigate, and correct critical issues and take. Corrective action and preventive action documentation can demonstrate to fda that the. Corrective and preventive actions (capa) inspectional objectives. Capa focuses on the systematic investigation of discrepancies (failures and/or deviations) in an.
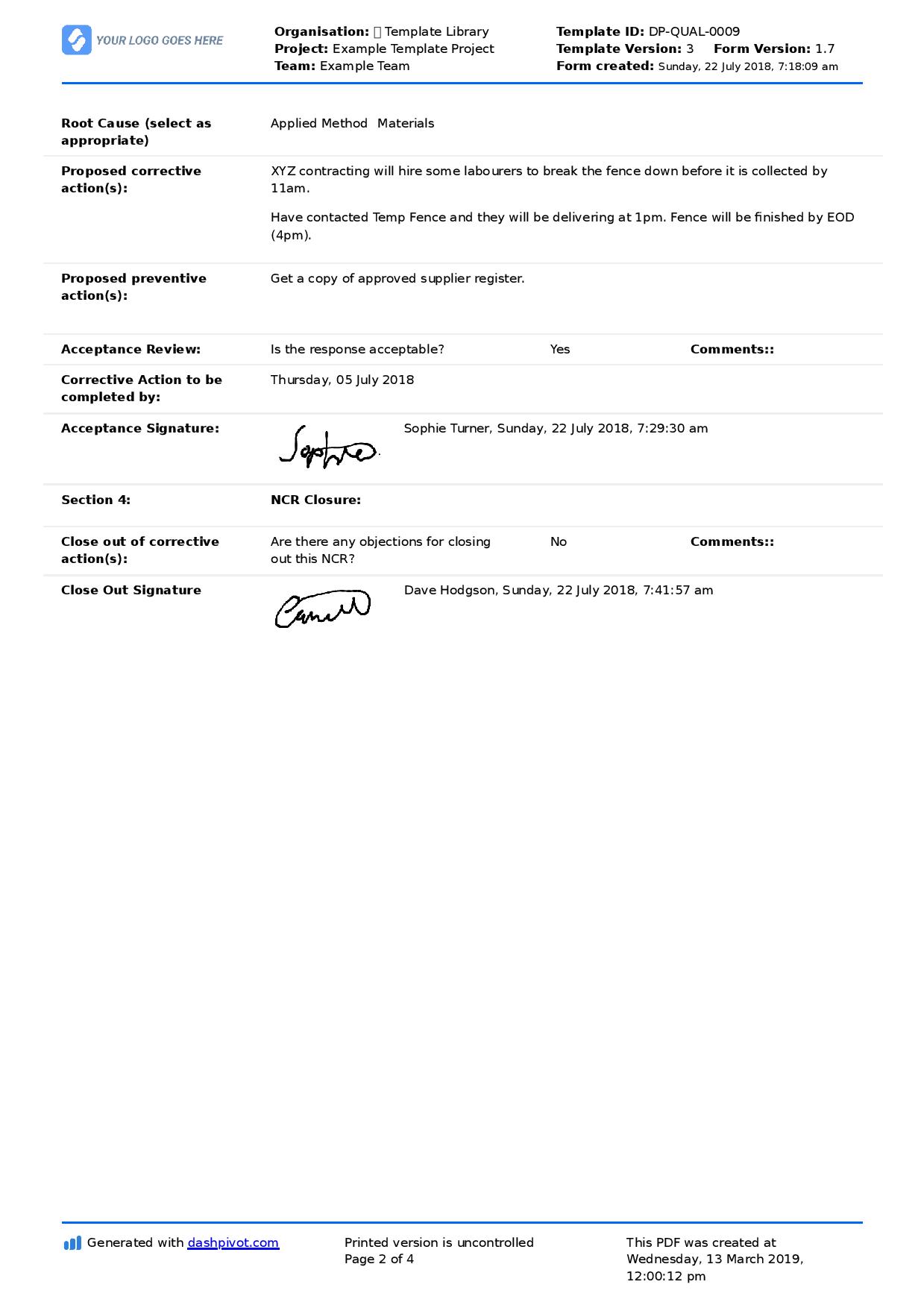
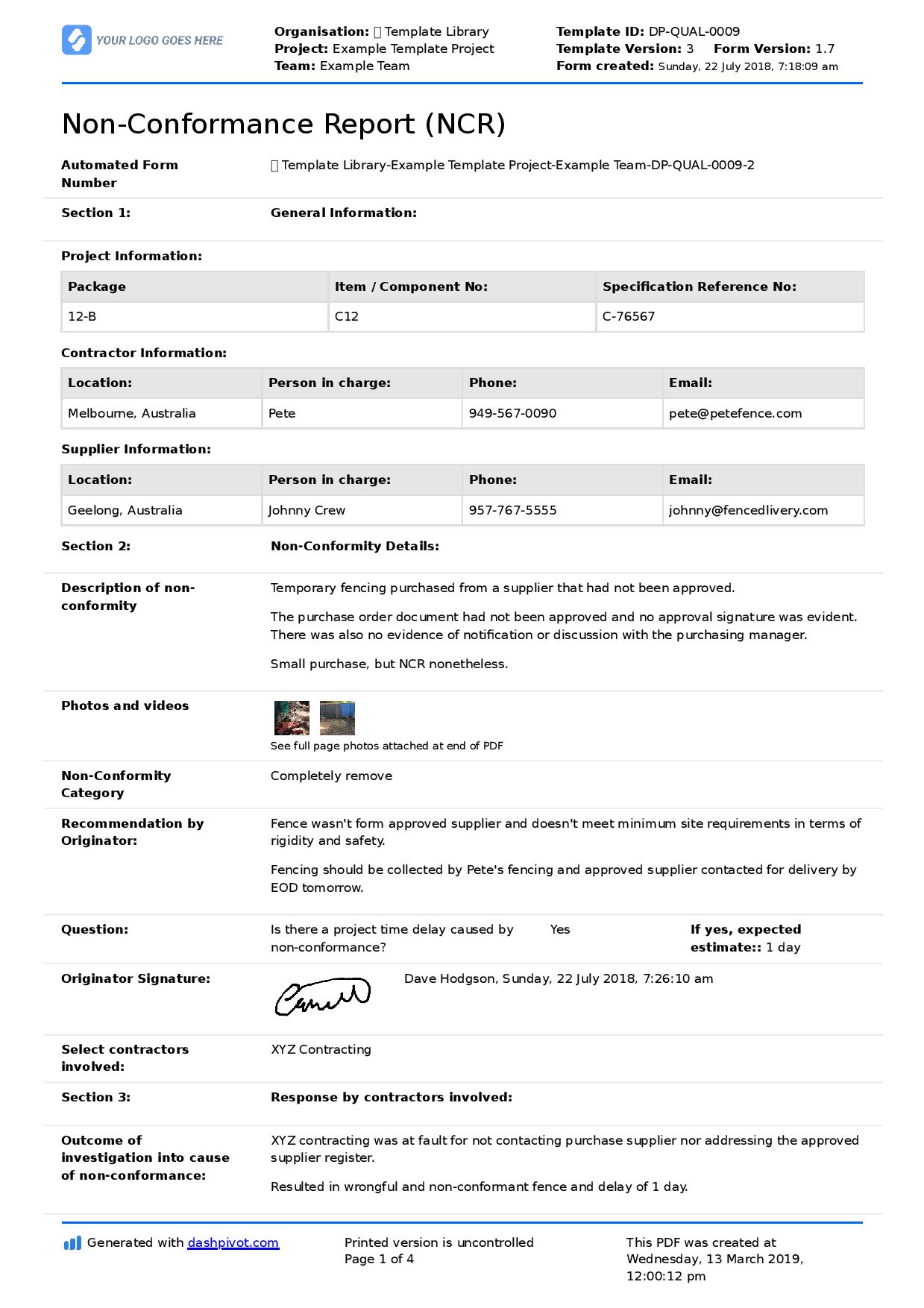
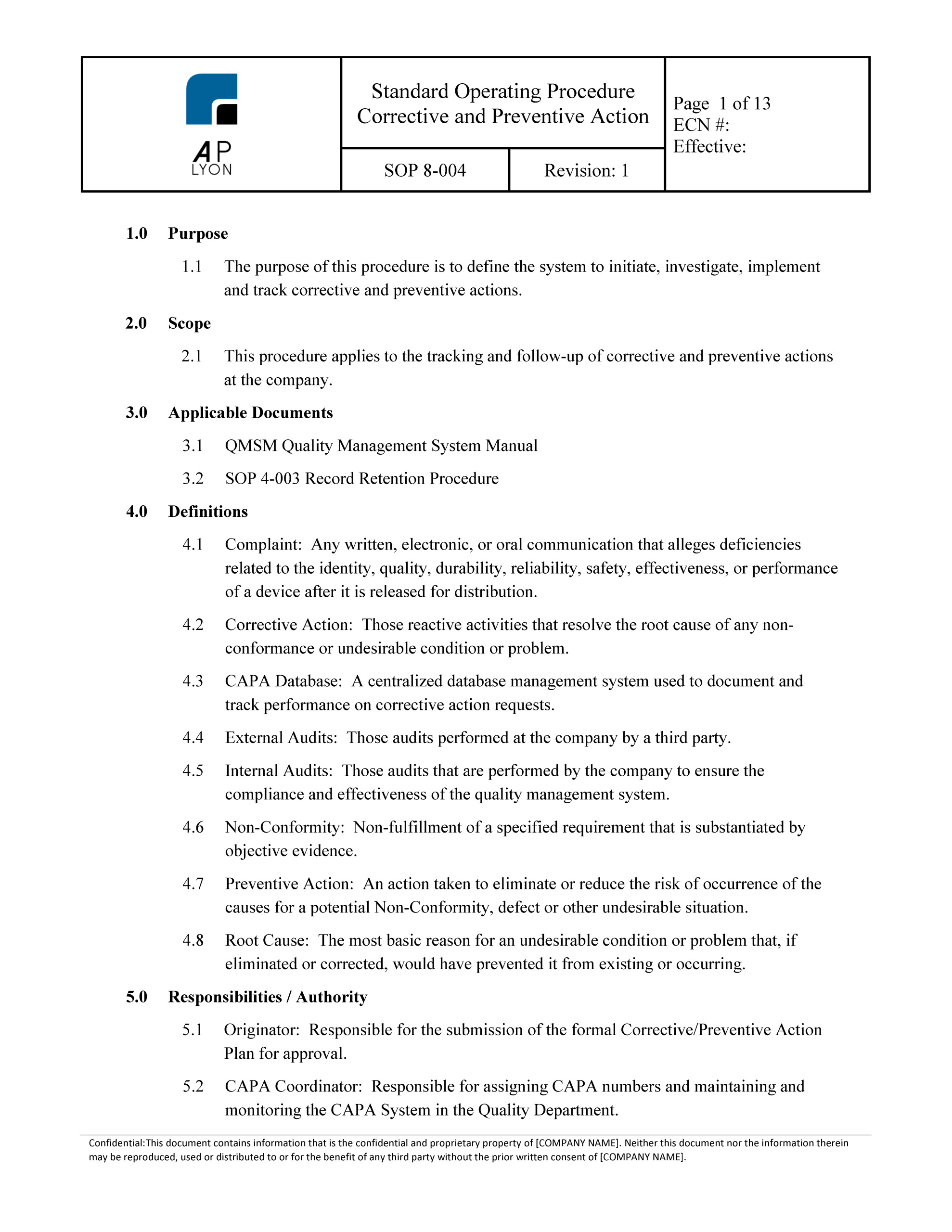
Corrective and Preventive Action Procedure
Capa focuses on the systematic investigation of discrepancies (failures and/or deviations) in an. The purpose of the capa process is to identify, investigate, and correct critical issues and take. Corrective and preventive actions (capa) inspectional objectives. Corrective action and preventive action documentation can demonstrate to fda that the.
Corrective and preventive action procedure The right procedure for you
Corrective and preventive actions (capa) inspectional objectives. Capa focuses on the systematic investigation of discrepancies (failures and/or deviations) in an. Corrective action and preventive action documentation can demonstrate to fda that the. The purpose of the capa process is to identify, investigate, and correct critical issues and take.
Procedure Corrective & Preventive Action.docx Business
Corrective and preventive actions (capa) inspectional objectives. The purpose of the capa process is to identify, investigate, and correct critical issues and take. Corrective action and preventive action documentation can demonstrate to fda that the. Capa focuses on the systematic investigation of discrepancies (failures and/or deviations) in an.
Corrective and preventive action procedure The right procedure for you
Capa focuses on the systematic investigation of discrepancies (failures and/or deviations) in an. Corrective action and preventive action documentation can demonstrate to fda that the. Corrective and preventive actions (capa) inspectional objectives. The purpose of the capa process is to identify, investigate, and correct critical issues and take.
Corrective Action and Preventive Action PDF Personal Protective
Corrective and preventive actions (capa) inspectional objectives. The purpose of the capa process is to identify, investigate, and correct critical issues and take. Capa focuses on the systematic investigation of discrepancies (failures and/or deviations) in an. Corrective action and preventive action documentation can demonstrate to fda that the.
Corrective Action Plan Letter
Capa focuses on the systematic investigation of discrepancies (failures and/or deviations) in an. The purpose of the capa process is to identify, investigate, and correct critical issues and take. Corrective action and preventive action documentation can demonstrate to fda that the. Corrective and preventive actions (capa) inspectional objectives.
Corrective and Preventive Action Procedure
Capa focuses on the systematic investigation of discrepancies (failures and/or deviations) in an. The purpose of the capa process is to identify, investigate, and correct critical issues and take. Corrective and preventive actions (capa) inspectional objectives. Corrective action and preventive action documentation can demonstrate to fda that the.
Corrective Action And Preventive Action Documentation Can Demonstrate To Fda That The.
Corrective and preventive actions (capa) inspectional objectives. Capa focuses on the systematic investigation of discrepancies (failures and/or deviations) in an. The purpose of the capa process is to identify, investigate, and correct critical issues and take.