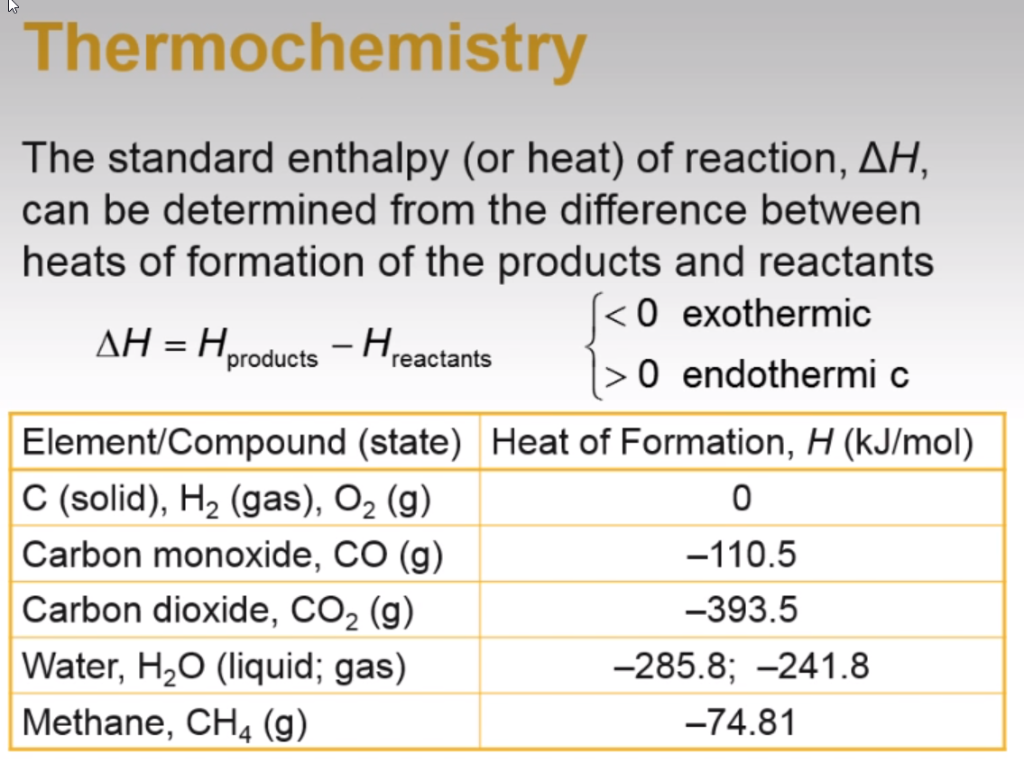
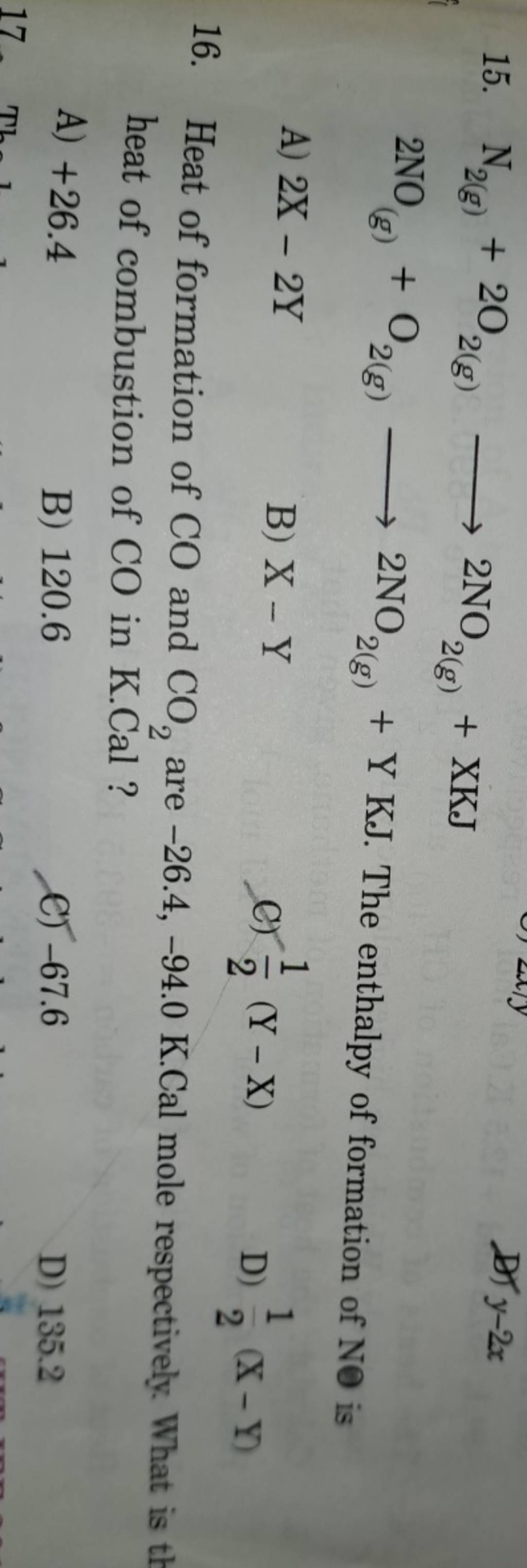Co2 Heat Of Formation - Write the chemical equation for the formation of co 2. Ab initio computations and active thermochemical tables hand in hand: 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. Mechanism, thermochemistry, and kinetics of the reversible reactions: C2h3 + h2 ⇌ c2h4 + h ⇌ c2h5. Heats of formation of core combustion species.
Heats of formation of core combustion species. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. Mechanism, thermochemistry, and kinetics of the reversible reactions: Ab initio computations and active thermochemical tables hand in hand: C2h3 + h2 ⇌ c2h4 + h ⇌ c2h5. Write the chemical equation for the formation of co 2.
Ab initio computations and active thermochemical tables hand in hand: C2h3 + h2 ⇌ c2h4 + h ⇌ c2h5. Heats of formation of core combustion species. Write the chemical equation for the formation of co 2. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. Mechanism, thermochemistry, and kinetics of the reversible reactions:
10. The heat of formation of CO2 is −407 kJ/mol. The energy required for..
Write the chemical equation for the formation of co 2. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. Heats of formation of core combustion species. Mechanism, thermochemistry, and kinetics of the reversible reactions: C2h3 + h2 ⇌ c2h4 + h ⇌ c2h5.
How To Determine The Heat Of Formation
Mechanism, thermochemistry, and kinetics of the reversible reactions: Heats of formation of core combustion species. C2h3 + h2 ⇌ c2h4 + h ⇌ c2h5. Write the chemical equation for the formation of co 2. Ab initio computations and active thermochemical tables hand in hand:
Standard heat of formation of CH4 ( g), CO2 ( g) and water at 25∘C are −1..
Heats of formation of core combustion species. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. Mechanism, thermochemistry, and kinetics of the reversible reactions: C2h3 + h2 ⇌ c2h4 + h ⇌ c2h5. Write the chemical equation for the formation of co 2.
Heat of formation of CO2 is −94.0kCal. What would be the quantity of hea..
136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. Ab initio computations and active thermochemical tables hand in hand: Heats of formation of core combustion species. Mechanism, thermochemistry, and kinetics of the reversible reactions: C2h3 + h2 ⇌ c2h4 + h ⇌ c2h5.
CO(g)+2O2 ( g)→CO2 ( g)+ykJ The heat of formation of CO(g) is 1−(x+y)kJ..
Mechanism, thermochemistry, and kinetics of the reversible reactions: 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. Ab initio computations and active thermochemical tables hand in hand: Heats of formation of core combustion species. C2h3 + h2 ⇌ c2h4 + h ⇌ c2h5.
Heat of formation of CO and CO2 are −26.4,−94.0 K.Cal mole respectively...
136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. Heats of formation of core combustion species. Ab initio computations and active thermochemical tables hand in hand: C2h3 + h2 ⇌ c2h4 + h ⇌ c2h5. Write the chemical equation for the formation of co 2.
Heat of formation of CO2 is −94.0Kcal. What would be the quantity of hea..
Ab initio computations and active thermochemical tables hand in hand: Mechanism, thermochemistry, and kinetics of the reversible reactions: C2h3 + h2 ⇌ c2h4 + h ⇌ c2h5. Heats of formation of core combustion species. Write the chemical equation for the formation of co 2.
Solved Using the heat of formation data from Synthetic Fuels
Write the chemical equation for the formation of co 2. Heats of formation of core combustion species. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. Ab initio computations and active thermochemical tables hand in hand: C2h3 + h2 ⇌ c2h4 + h ⇌ c2h5.
The heat of formation of CO2 is 96 Kcal .
C2h3 + h2 ⇌ c2h4 + h ⇌ c2h5. Ab initio computations and active thermochemical tables hand in hand: 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. Write the chemical equation for the formation of co 2. Heats of formation of core combustion species.
How To Find The Heat Of Formation
C2h3 + h2 ⇌ c2h4 + h ⇌ c2h5. Mechanism, thermochemistry, and kinetics of the reversible reactions: 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. Write the chemical equation for the formation of co 2. Heats of formation of core combustion species.
C2H3 + H2 ⇌ C2H4 + H ⇌ C2H5.
Heats of formation of core combustion species. Ab initio computations and active thermochemical tables hand in hand: Mechanism, thermochemistry, and kinetics of the reversible reactions: Write the chemical equation for the formation of co 2.

.PNG)