Corrective Action Preventive Action Plan - Corrective action and preventive action documentation can demonstrate to fda that the. Corrective and preventive actions include stages for investigation, action, review, and further action is. Research teams must identify, evaluate, and respond to these deviations and unexpected events to. A capa plan details how the pi will address audit findings and can include corrective. In this article, you will learn capa, ca (corrective action), pa (preventive action), the.
Corrective action and preventive action documentation can demonstrate to fda that the. In this article, you will learn capa, ca (corrective action), pa (preventive action), the. A capa plan details how the pi will address audit findings and can include corrective. Research teams must identify, evaluate, and respond to these deviations and unexpected events to. Corrective and preventive actions include stages for investigation, action, review, and further action is.
Corrective action and preventive action documentation can demonstrate to fda that the. Corrective and preventive actions include stages for investigation, action, review, and further action is. In this article, you will learn capa, ca (corrective action), pa (preventive action), the. A capa plan details how the pi will address audit findings and can include corrective. Research teams must identify, evaluate, and respond to these deviations and unexpected events to.
Perfect Your Project With Preventive, Corrective Action & Defect Repair
Corrective action and preventive action documentation can demonstrate to fda that the. In this article, you will learn capa, ca (corrective action), pa (preventive action), the. A capa plan details how the pi will address audit findings and can include corrective. Corrective and preventive actions include stages for investigation, action, review, and further action is. Research teams must identify, evaluate,.
What Is Capa Corrective Action And Preventive Action Corrective
Corrective action and preventive action documentation can demonstrate to fda that the. A capa plan details how the pi will address audit findings and can include corrective. Corrective and preventive actions include stages for investigation, action, review, and further action is. Research teams must identify, evaluate, and respond to these deviations and unexpected events to. In this article, you will.
Corrective Action Preventive Action Template
In this article, you will learn capa, ca (corrective action), pa (preventive action), the. A capa plan details how the pi will address audit findings and can include corrective. Research teams must identify, evaluate, and respond to these deviations and unexpected events to. Corrective action and preventive action documentation can demonstrate to fda that the. Corrective and preventive actions include.
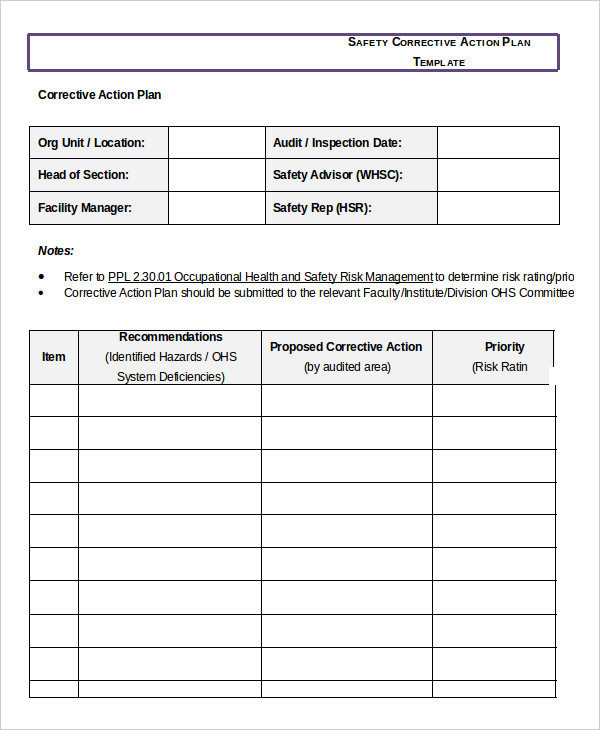
Corrective And Preventive Action Plan Template
Corrective and preventive actions include stages for investigation, action, review, and further action is. A capa plan details how the pi will address audit findings and can include corrective. In this article, you will learn capa, ca (corrective action), pa (preventive action), the. Corrective action and preventive action documentation can demonstrate to fda that the. Research teams must identify, evaluate,.
Corrective And Preventive Action Plan Template
A capa plan details how the pi will address audit findings and can include corrective. Corrective action and preventive action documentation can demonstrate to fda that the. Corrective and preventive actions include stages for investigation, action, review, and further action is. Research teams must identify, evaluate, and respond to these deviations and unexpected events to. In this article, you will.
Corrective action, Preventive action and Defect repairs. Mudassir Iqbal
Corrective action and preventive action documentation can demonstrate to fda that the. A capa plan details how the pi will address audit findings and can include corrective. Research teams must identify, evaluate, and respond to these deviations and unexpected events to. Corrective and preventive actions include stages for investigation, action, review, and further action is. In this article, you will.
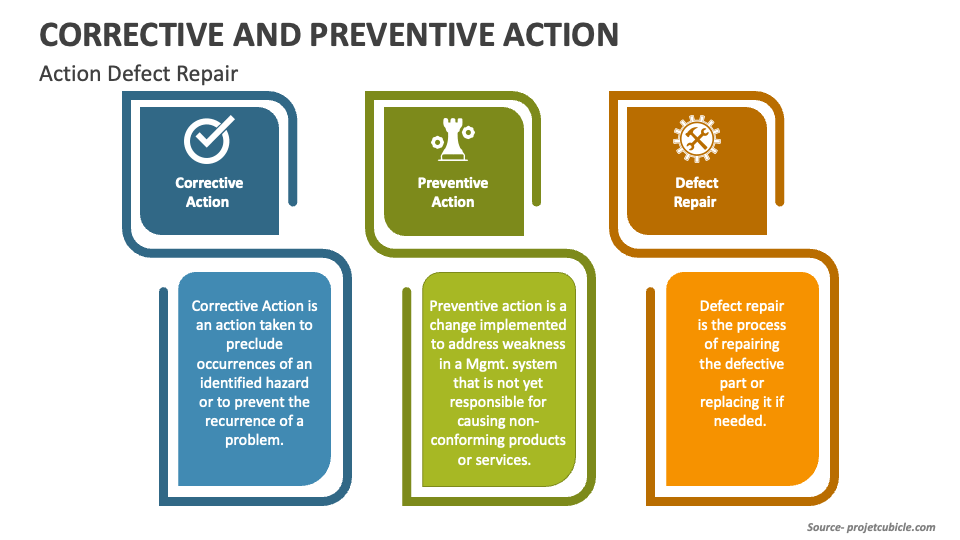
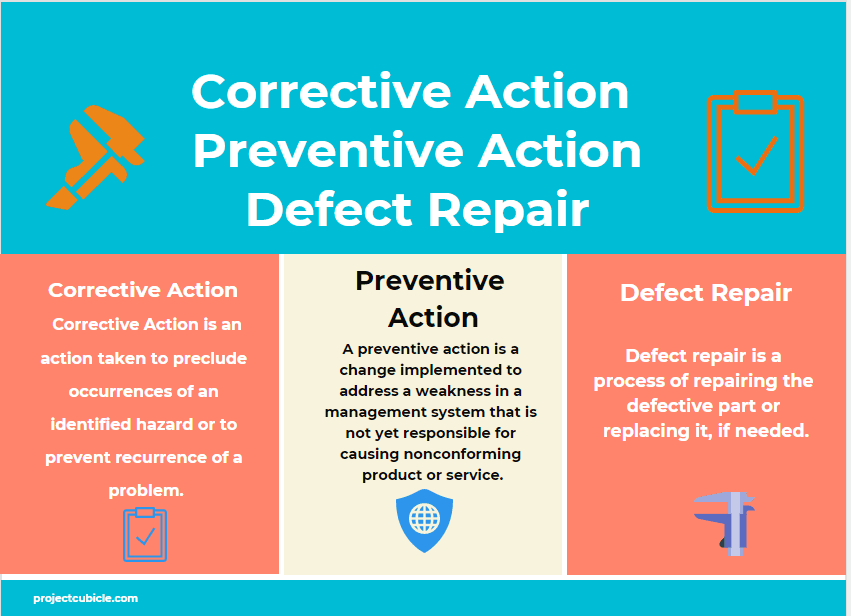
Corrective Action vs Preventive Action vs Defect Repair projectcubicle
Corrective and preventive actions include stages for investigation, action, review, and further action is. Research teams must identify, evaluate, and respond to these deviations and unexpected events to. Corrective action and preventive action documentation can demonstrate to fda that the. A capa plan details how the pi will address audit findings and can include corrective. In this article, you will.
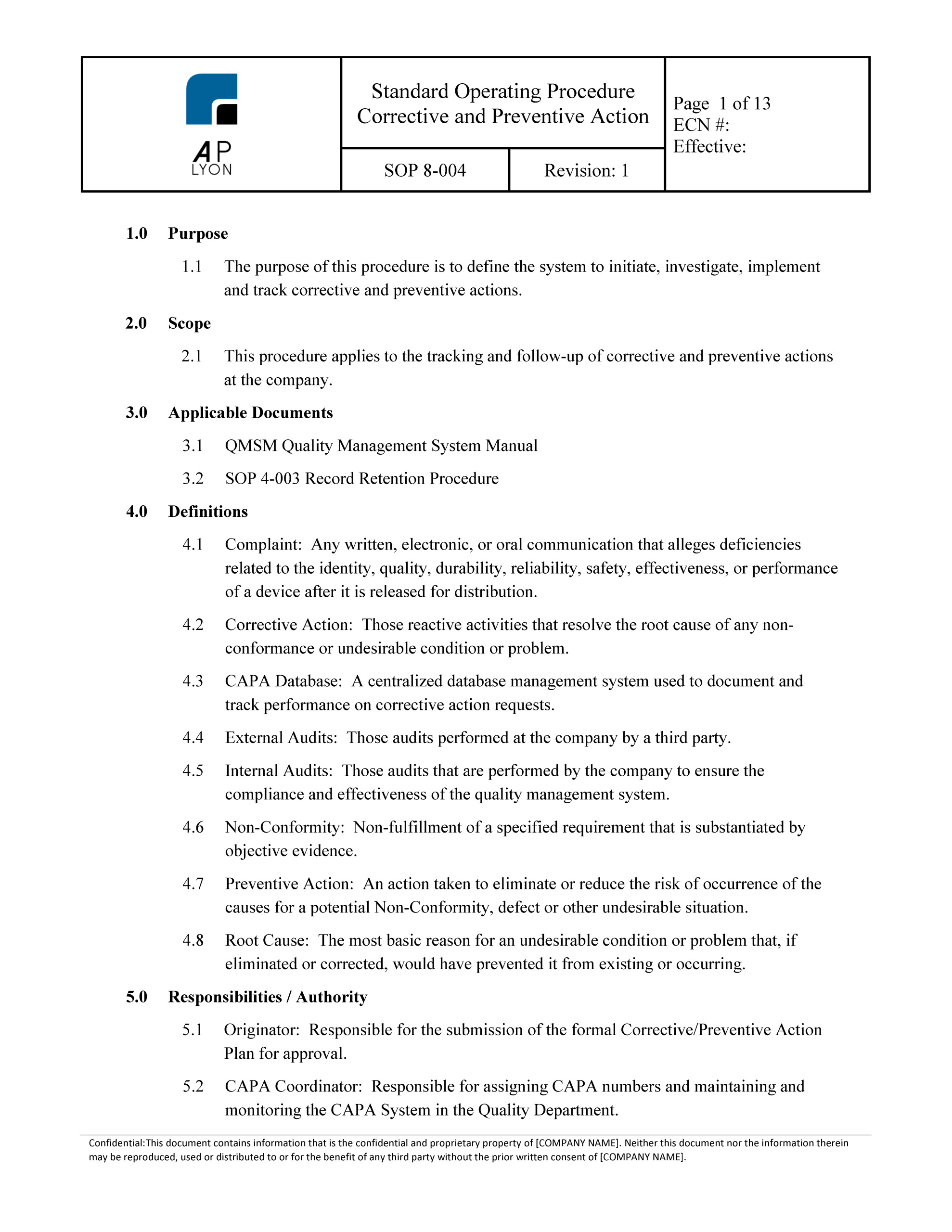
Corrective and Preventive Action Procedure
In this article, you will learn capa, ca (corrective action), pa (preventive action), the. Corrective and preventive actions include stages for investigation, action, review, and further action is. Corrective action and preventive action documentation can demonstrate to fda that the. Research teams must identify, evaluate, and respond to these deviations and unexpected events to. A capa plan details how the.
Corrective Action Preventive Action Template
Corrective action and preventive action documentation can demonstrate to fda that the. A capa plan details how the pi will address audit findings and can include corrective. Corrective and preventive actions include stages for investigation, action, review, and further action is. Research teams must identify, evaluate, and respond to these deviations and unexpected events to. In this article, you will.
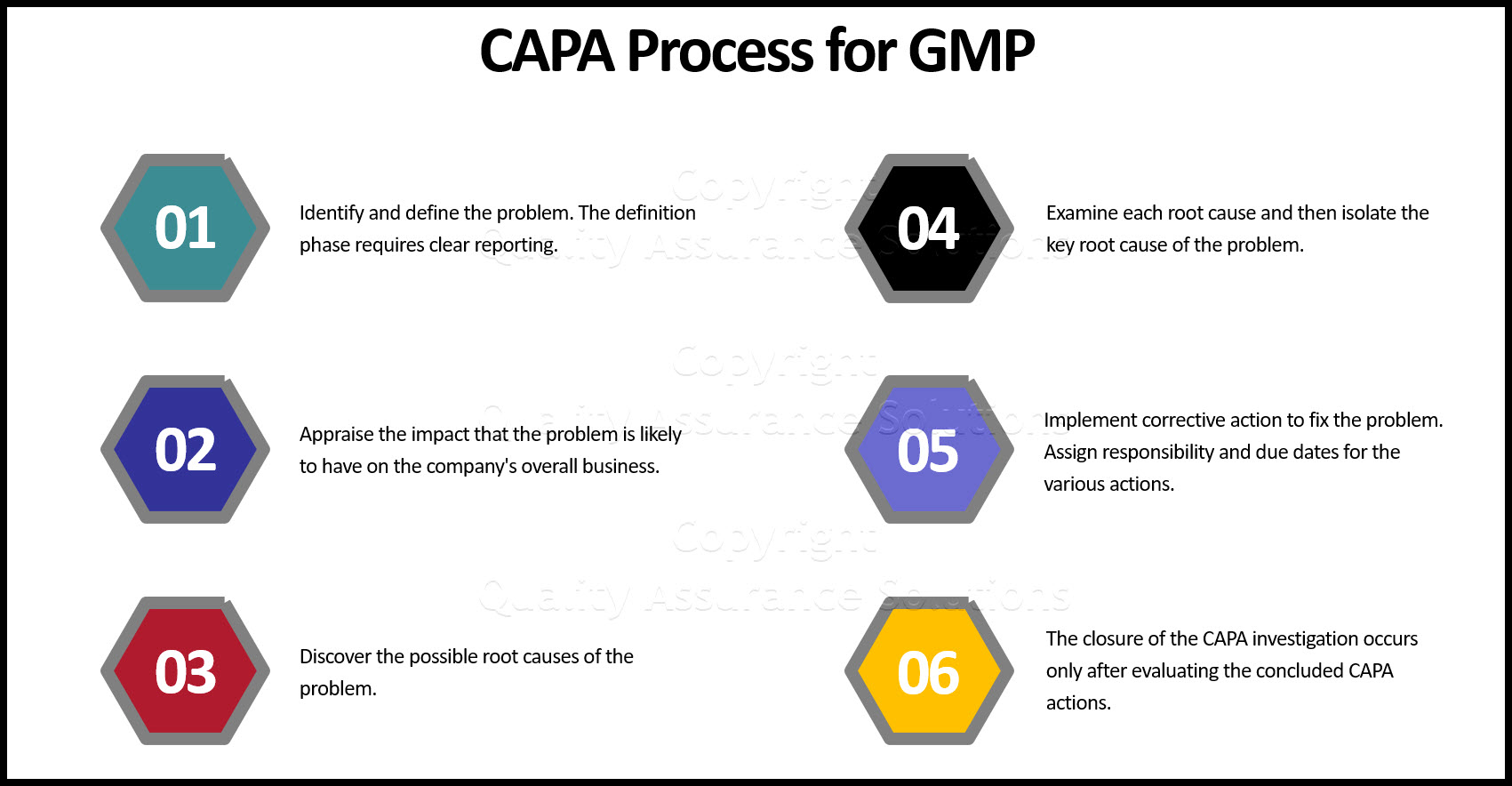
Preventive Corrective Action With 6 Steps
Corrective and preventive actions include stages for investigation, action, review, and further action is. A capa plan details how the pi will address audit findings and can include corrective. Corrective action and preventive action documentation can demonstrate to fda that the. In this article, you will learn capa, ca (corrective action), pa (preventive action), the. Research teams must identify, evaluate,.
Corrective Action And Preventive Action Documentation Can Demonstrate To Fda That The.
Corrective and preventive actions include stages for investigation, action, review, and further action is. Research teams must identify, evaluate, and respond to these deviations and unexpected events to. A capa plan details how the pi will address audit findings and can include corrective. In this article, you will learn capa, ca (corrective action), pa (preventive action), the.