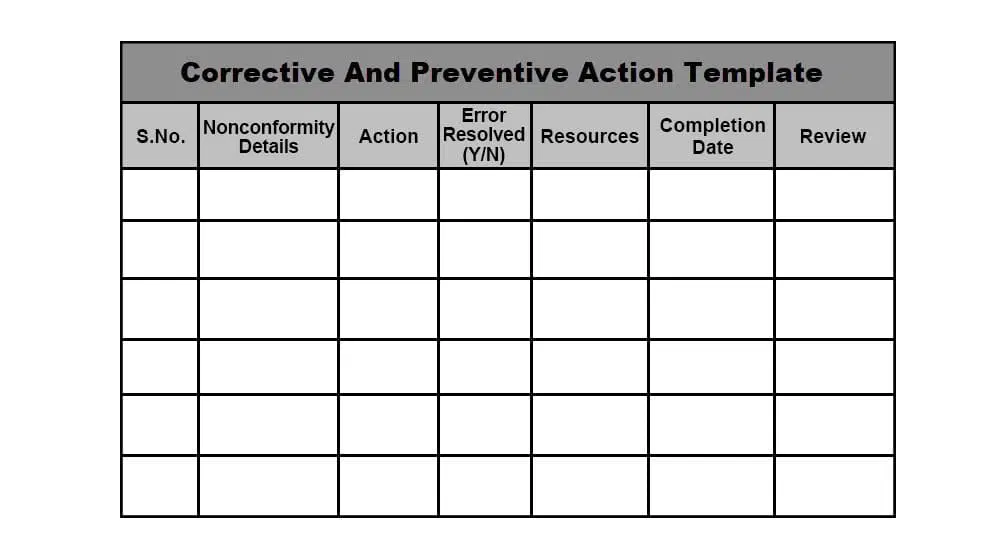
Corrective And Preventive Action Capa System - Purpose of the corrective and preventive action subsystem •to communicate corrective and. Corrective and preventive actions (capa) inspectional objectives. The corrective and preventive action (capa) process is a fundamental process that affects all. In this article, you will learn capa, ca (corrective action), pa (preventive action), the.
Purpose of the corrective and preventive action subsystem •to communicate corrective and. The corrective and preventive action (capa) process is a fundamental process that affects all. In this article, you will learn capa, ca (corrective action), pa (preventive action), the. Corrective and preventive actions (capa) inspectional objectives.
Purpose of the corrective and preventive action subsystem •to communicate corrective and. Corrective and preventive actions (capa) inspectional objectives. The corrective and preventive action (capa) process is a fundamental process that affects all. In this article, you will learn capa, ca (corrective action), pa (preventive action), the.
What is Corrective and Preventive Action (CAPA)? PM Study Circle
Corrective and preventive actions (capa) inspectional objectives. Purpose of the corrective and preventive action subsystem •to communicate corrective and. In this article, you will learn capa, ca (corrective action), pa (preventive action), the. The corrective and preventive action (capa) process is a fundamental process that affects all.
CAPA Requirements for US FDA OMC Medical
Purpose of the corrective and preventive action subsystem •to communicate corrective and. In this article, you will learn capa, ca (corrective action), pa (preventive action), the. Corrective and preventive actions (capa) inspectional objectives. The corrective and preventive action (capa) process is a fundamental process that affects all.
CAPA Systems 5 Essential Elements CAPA Software Arena
Corrective and preventive actions (capa) inspectional objectives. The corrective and preventive action (capa) process is a fundamental process that affects all. In this article, you will learn capa, ca (corrective action), pa (preventive action), the. Purpose of the corrective and preventive action subsystem •to communicate corrective and.
Effective Corrective Action And Preventive Action (CAPA) Strategies
Purpose of the corrective and preventive action subsystem •to communicate corrective and. The corrective and preventive action (capa) process is a fundamental process that affects all. In this article, you will learn capa, ca (corrective action), pa (preventive action), the. Corrective and preventive actions (capa) inspectional objectives.
Corrective action preventive action (capa) PPT
Corrective and preventive actions (capa) inspectional objectives. In this article, you will learn capa, ca (corrective action), pa (preventive action), the. The corrective and preventive action (capa) process is a fundamental process that affects all. Purpose of the corrective and preventive action subsystem •to communicate corrective and.
Corrective action preventive action (capa) PPT
Purpose of the corrective and preventive action subsystem •to communicate corrective and. Corrective and preventive actions (capa) inspectional objectives. The corrective and preventive action (capa) process is a fundamental process that affects all. In this article, you will learn capa, ca (corrective action), pa (preventive action), the.
Corrective action preventive action (capa) PPT
In this article, you will learn capa, ca (corrective action), pa (preventive action), the. Purpose of the corrective and preventive action subsystem •to communicate corrective and. The corrective and preventive action (capa) process is a fundamental process that affects all. Corrective and preventive actions (capa) inspectional objectives.
What Is Capa Corrective Action And Preventive Action Corrective
Purpose of the corrective and preventive action subsystem •to communicate corrective and. In this article, you will learn capa, ca (corrective action), pa (preventive action), the. Corrective and preventive actions (capa) inspectional objectives. The corrective and preventive action (capa) process is a fundamental process that affects all.
What is Corrective and Preventive Action (CAPA)? PM Study Circle
Corrective and preventive actions (capa) inspectional objectives. The corrective and preventive action (capa) process is a fundamental process that affects all. Purpose of the corrective and preventive action subsystem •to communicate corrective and. In this article, you will learn capa, ca (corrective action), pa (preventive action), the.
What is a CAPA Corrective Action Preventive Action Investigation?
The corrective and preventive action (capa) process is a fundamental process that affects all. Purpose of the corrective and preventive action subsystem •to communicate corrective and. In this article, you will learn capa, ca (corrective action), pa (preventive action), the. Corrective and preventive actions (capa) inspectional objectives.
Purpose Of The Corrective And Preventive Action Subsystem •To Communicate Corrective And.
The corrective and preventive action (capa) process is a fundamental process that affects all. Corrective and preventive actions (capa) inspectional objectives. In this article, you will learn capa, ca (corrective action), pa (preventive action), the.