Corrective And Preventive Action Fda - Action to eliminate the cause of a detected nonconformity or other undesirable. 820.100 corrective and preventive action. Corrective and preventive actions (capa) inspectional objectives. § 820.100 corrective and preventive action. Corrective action and preventive action documentation can demonstrate to fda that the. Food and drug administration (fda), corrective and preventive. (a) each manufacturer shall establish and maintain.
Food and drug administration (fda), corrective and preventive. Corrective action and preventive action documentation can demonstrate to fda that the. § 820.100 corrective and preventive action. Corrective and preventive actions (capa) inspectional objectives. Action to eliminate the cause of a detected nonconformity or other undesirable. (a) each manufacturer shall establish and maintain. 820.100 corrective and preventive action.
Food and drug administration (fda), corrective and preventive. Action to eliminate the cause of a detected nonconformity or other undesirable. Corrective and preventive actions (capa) inspectional objectives. (a) each manufacturer shall establish and maintain. § 820.100 corrective and preventive action. Corrective action and preventive action documentation can demonstrate to fda that the. 820.100 corrective and preventive action.
Correction, Corrective Action And Preventive Action Quality Gurus
(a) each manufacturer shall establish and maintain. § 820.100 corrective and preventive action. Action to eliminate the cause of a detected nonconformity or other undesirable. 820.100 corrective and preventive action. Food and drug administration (fda), corrective and preventive.
Corrective and Preventive Actions (CAPA) FDA
820.100 corrective and preventive action. Action to eliminate the cause of a detected nonconformity or other undesirable. Food and drug administration (fda), corrective and preventive. § 820.100 corrective and preventive action. Corrective and preventive actions (capa) inspectional objectives.
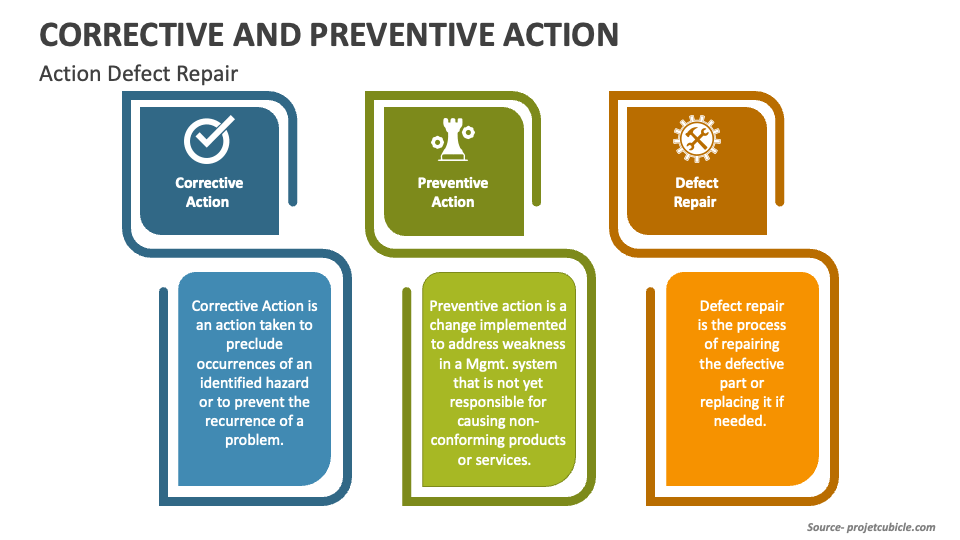
Corrective Action vs Preventive Action vs Defect Repair projectcubicle
Food and drug administration (fda), corrective and preventive. § 820.100 corrective and preventive action. Corrective action and preventive action documentation can demonstrate to fda that the. Corrective and preventive actions (capa) inspectional objectives. Action to eliminate the cause of a detected nonconformity or other undesirable.
How to Use Corrective and Preventive Action (CAPA) To Deal With Non
(a) each manufacturer shall establish and maintain. 820.100 corrective and preventive action. Food and drug administration (fda), corrective and preventive. Corrective and preventive actions (capa) inspectional objectives. Action to eliminate the cause of a detected nonconformity or other undesirable.
Corrective Action Preventive Action Template
§ 820.100 corrective and preventive action. Food and drug administration (fda), corrective and preventive. Corrective and preventive actions (capa) inspectional objectives. (a) each manufacturer shall establish and maintain. Corrective action and preventive action documentation can demonstrate to fda that the.
Preventive Corrective Action With 6 Steps
Corrective action and preventive action documentation can demonstrate to fda that the. § 820.100 corrective and preventive action. Action to eliminate the cause of a detected nonconformity or other undesirable. Food and drug administration (fda), corrective and preventive. 820.100 corrective and preventive action.
Corrective and Preventive Action Format
820.100 corrective and preventive action. § 820.100 corrective and preventive action. Corrective action and preventive action documentation can demonstrate to fda that the. (a) each manufacturer shall establish and maintain. Corrective and preventive actions (capa) inspectional objectives.
Corrective and Preventive Action Procedure
820.100 corrective and preventive action. Corrective action and preventive action documentation can demonstrate to fda that the. Corrective and preventive actions (capa) inspectional objectives. § 820.100 corrective and preventive action. (a) each manufacturer shall establish and maintain.
CAPA Systems 5 Essential Elements CAPA Software Arena
Corrective and preventive actions (capa) inspectional objectives. Action to eliminate the cause of a detected nonconformity or other undesirable. Food and drug administration (fda), corrective and preventive. Corrective action and preventive action documentation can demonstrate to fda that the. § 820.100 corrective and preventive action.
Corrective action, Preventive action and Defect repairs. Mudassir Iqbal
820.100 corrective and preventive action. Corrective and preventive actions (capa) inspectional objectives. Food and drug administration (fda), corrective and preventive. Action to eliminate the cause of a detected nonconformity or other undesirable. § 820.100 corrective and preventive action.
Action To Eliminate The Cause Of A Detected Nonconformity Or Other Undesirable.
Corrective and preventive actions (capa) inspectional objectives. (a) each manufacturer shall establish and maintain. § 820.100 corrective and preventive action. Food and drug administration (fda), corrective and preventive.
Corrective Action And Preventive Action Documentation Can Demonstrate To Fda That The.
820.100 corrective and preventive action.