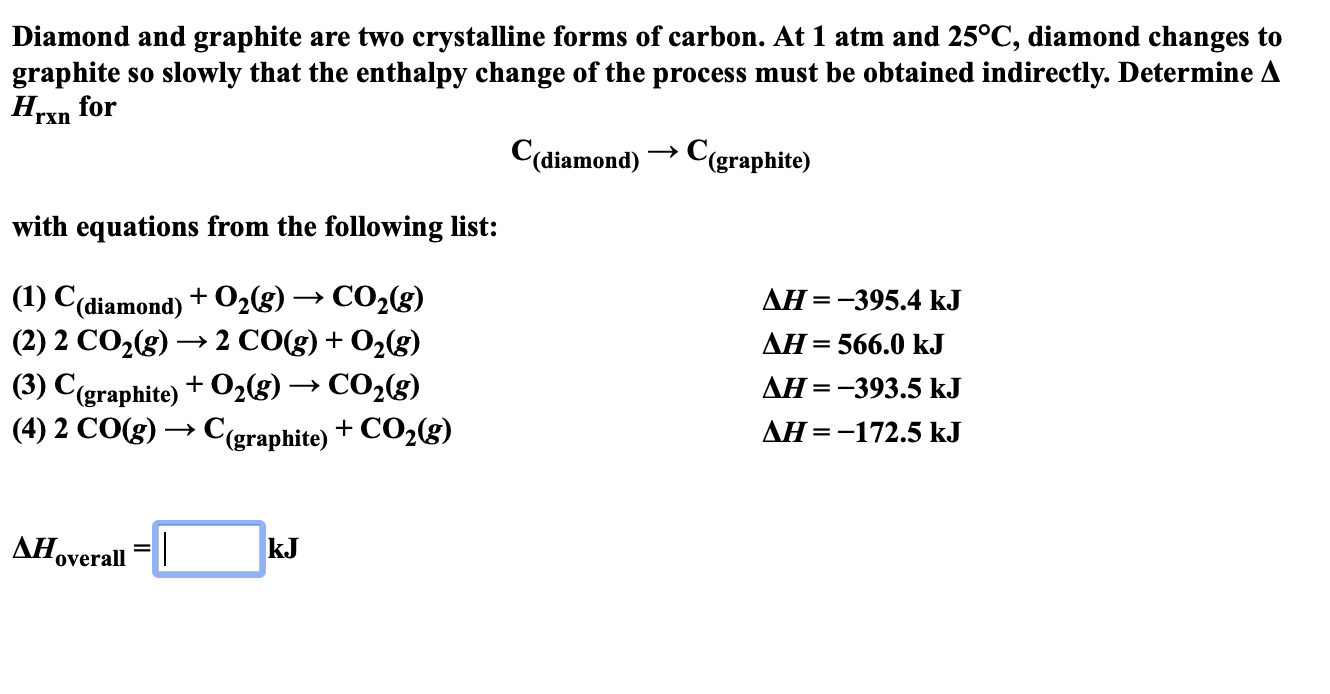
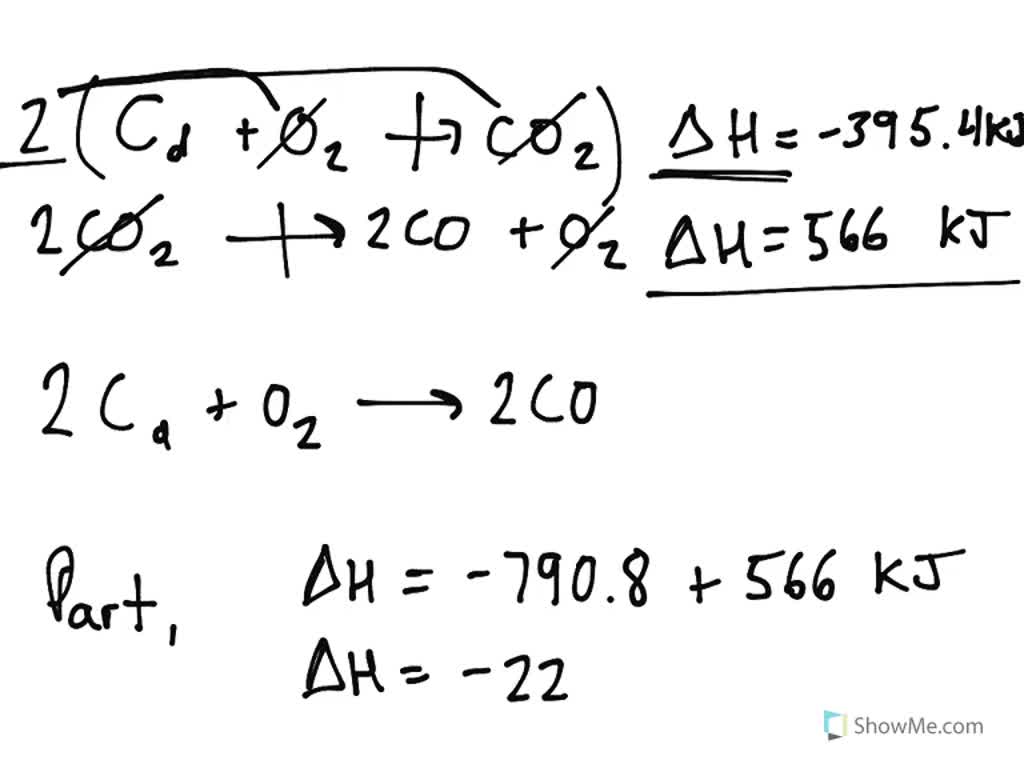Diamond And Graphite Are Two Crystalline Forms Of Carbon - Chemically, these minerals consist of carbon atoms with different physical properties. Diamond and graphite are called the allotropes of carbon. Diamond and graphite are two allotropes of carbon, meaning they are two different forms of the same element.
Diamond and graphite are two allotropes of carbon, meaning they are two different forms of the same element. Chemically, these minerals consist of carbon atoms with different physical properties. Diamond and graphite are called the allotropes of carbon.
Diamond and graphite are called the allotropes of carbon. Diamond and graphite are two allotropes of carbon, meaning they are two different forms of the same element. Chemically, these minerals consist of carbon atoms with different physical properties.
Solved Diamond and graphite are two crystalline forms of
Diamond and graphite are called the allotropes of carbon. Diamond and graphite are two allotropes of carbon, meaning they are two different forms of the same element. Chemically, these minerals consist of carbon atoms with different physical properties.
SOLVED Diamond and graphite are two crystalline forms of carbon. At 1
Diamond and graphite are two allotropes of carbon, meaning they are two different forms of the same element. Diamond and graphite are called the allotropes of carbon. Chemically, these minerals consist of carbon atoms with different physical properties.
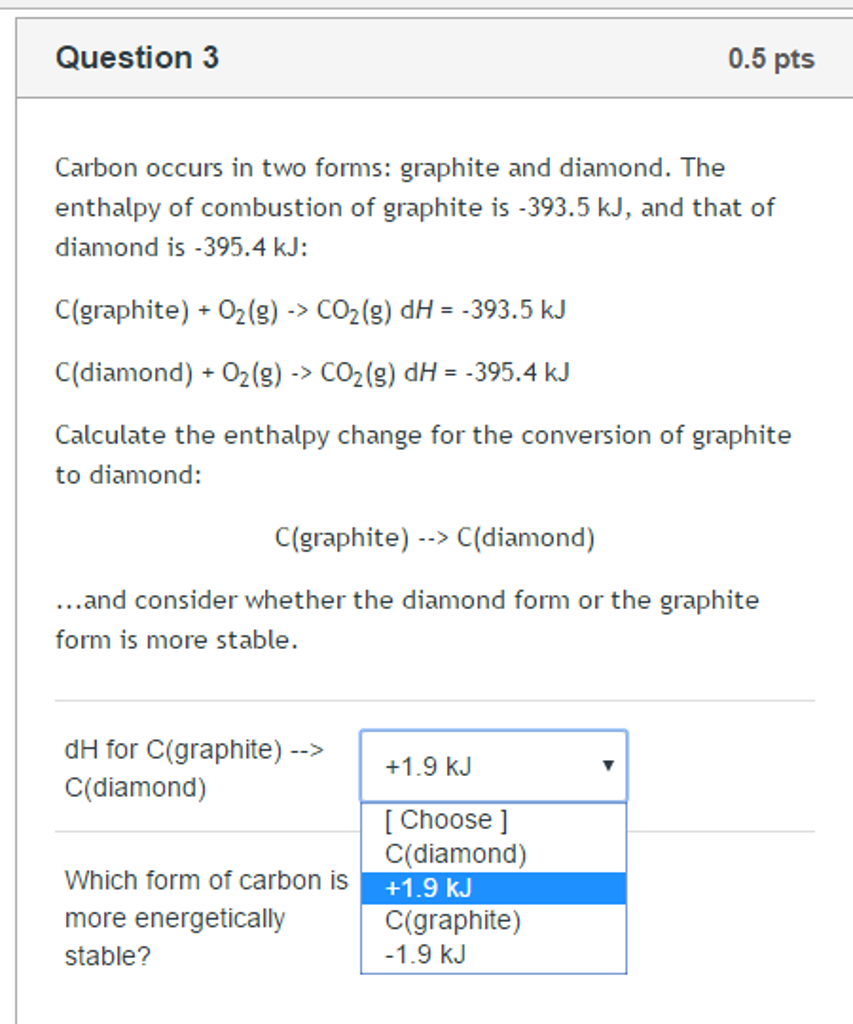
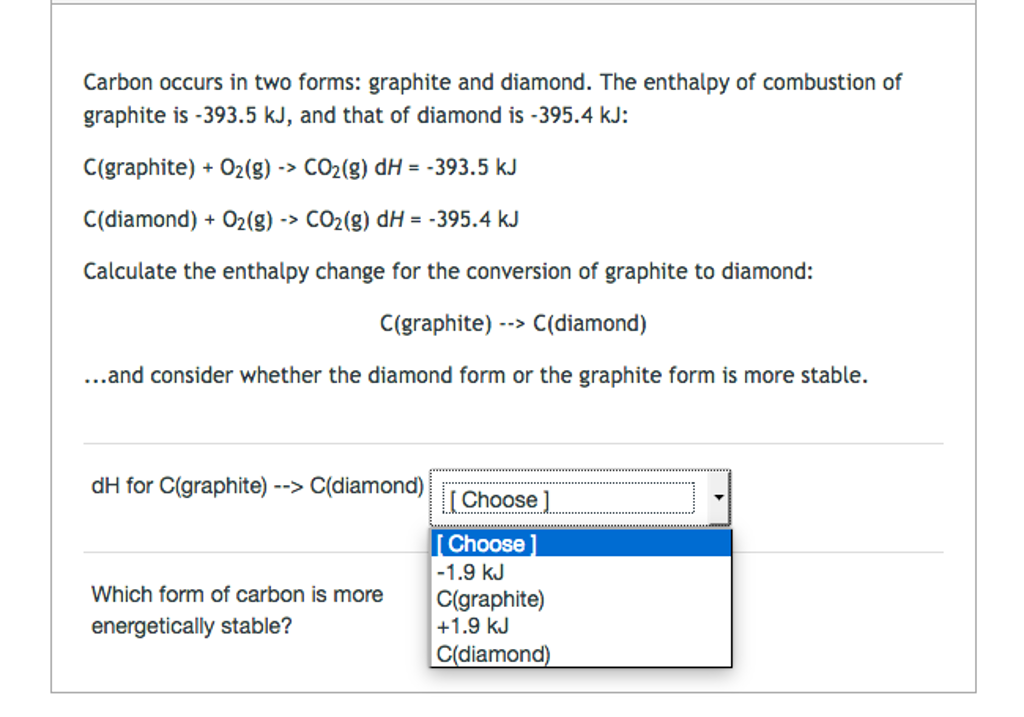
Solved Carbon occurs in two forms graphite and
Chemically, these minerals consist of carbon atoms with different physical properties. Diamond and graphite are called the allotropes of carbon. Diamond and graphite are two allotropes of carbon, meaning they are two different forms of the same element.
Solved Carbon occurs in two forms graphite and diamond. The
Chemically, these minerals consist of carbon atoms with different physical properties. Diamond and graphite are called the allotropes of carbon. Diamond and graphite are two allotropes of carbon, meaning they are two different forms of the same element.
SOLUTION Forms of crystalline carbon Studypool
Chemically, these minerals consist of carbon atoms with different physical properties. Diamond and graphite are called the allotropes of carbon. Diamond and graphite are two allotropes of carbon, meaning they are two different forms of the same element.
Solved Diamond and graphite are two crystalline forms of
Diamond and graphite are called the allotropes of carbon. Diamond and graphite are two allotropes of carbon, meaning they are two different forms of the same element. Chemically, these minerals consist of carbon atoms with different physical properties.
Allotropes of Carbon Forms of Carbon Giant Covalent Compounds
Diamond and graphite are called the allotropes of carbon. Chemically, these minerals consist of carbon atoms with different physical properties. Diamond and graphite are two allotropes of carbon, meaning they are two different forms of the same element.
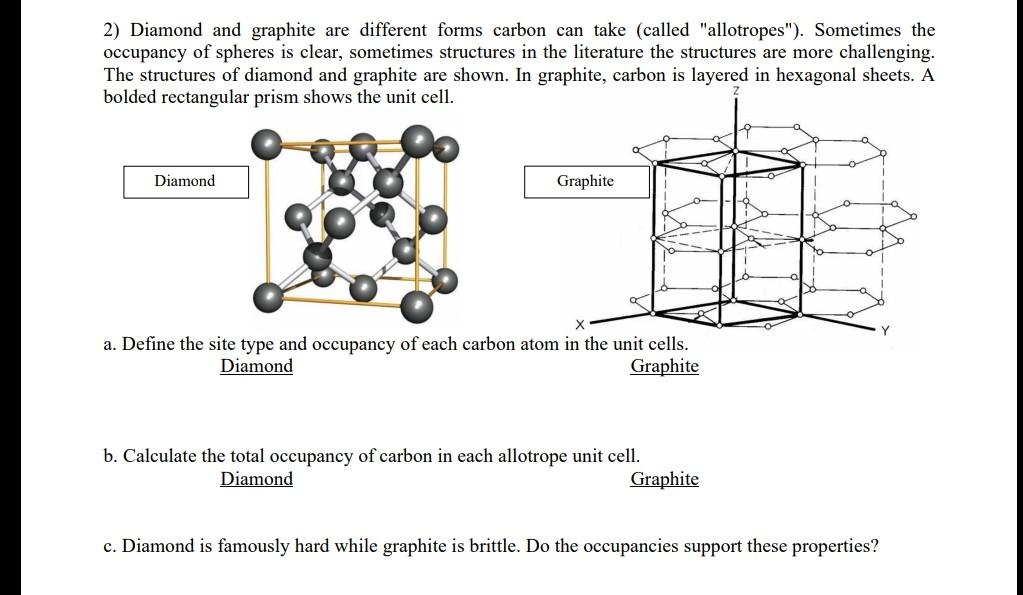
Solved 2) Diamond and graphite are different forms carbon
Chemically, these minerals consist of carbon atoms with different physical properties. Diamond and graphite are called the allotropes of carbon. Diamond and graphite are two allotropes of carbon, meaning they are two different forms of the same element.
CHEM Different Forms of Carbon Graphite vs. Diamond
Chemically, these minerals consist of carbon atoms with different physical properties. Diamond and graphite are called the allotropes of carbon. Diamond and graphite are two allotropes of carbon, meaning they are two different forms of the same element.
Answered Diamond and graphite are two… bartleby
Diamond and graphite are two allotropes of carbon, meaning they are two different forms of the same element. Diamond and graphite are called the allotropes of carbon. Chemically, these minerals consist of carbon atoms with different physical properties.
Diamond And Graphite Are Called The Allotropes Of Carbon.
Diamond and graphite are two allotropes of carbon, meaning they are two different forms of the same element. Chemically, these minerals consist of carbon atoms with different physical properties.