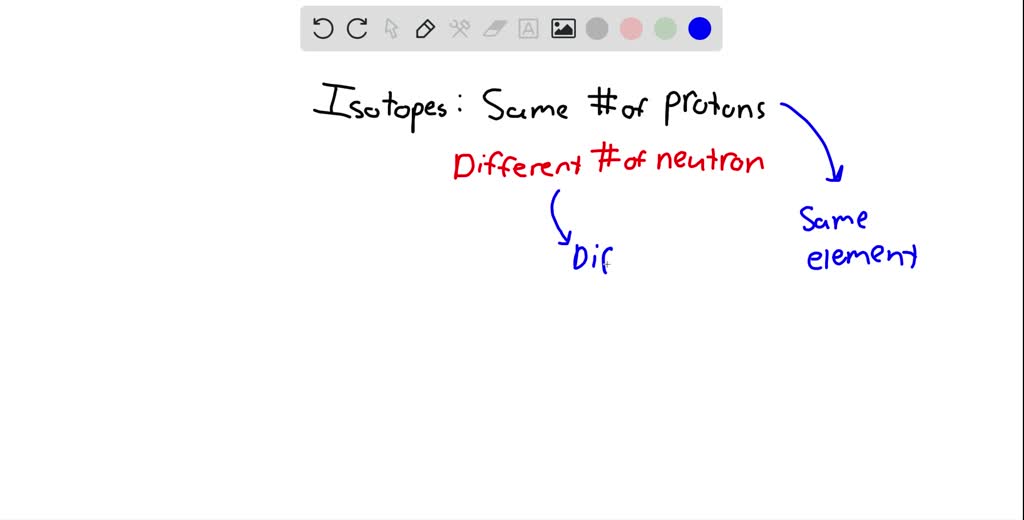
Different Forms Of Same Element - Isotopes have the same number of positive particles, called protons, and. Different forms of the same element are called isotopes. Allotropy describes how elements like carbon or oxygen can exist in different structural forms within the same physical.
Allotropy describes how elements like carbon or oxygen can exist in different structural forms within the same physical. Isotopes have the same number of positive particles, called protons, and. Different forms of the same element are called isotopes.
Different forms of the same element are called isotopes. Isotopes have the same number of positive particles, called protons, and. Allotropy describes how elements like carbon or oxygen can exist in different structural forms within the same physical.
SOLVEDAre all atoms of the same element identical? If not, how do they
Isotopes have the same number of positive particles, called protons, and. Different forms of the same element are called isotopes. Allotropy describes how elements like carbon or oxygen can exist in different structural forms within the same physical.
They are the same element r/ProgrammerHumor
Allotropy describes how elements like carbon or oxygen can exist in different structural forms within the same physical. Isotopes have the same number of positive particles, called protons, and. Different forms of the same element are called isotopes.
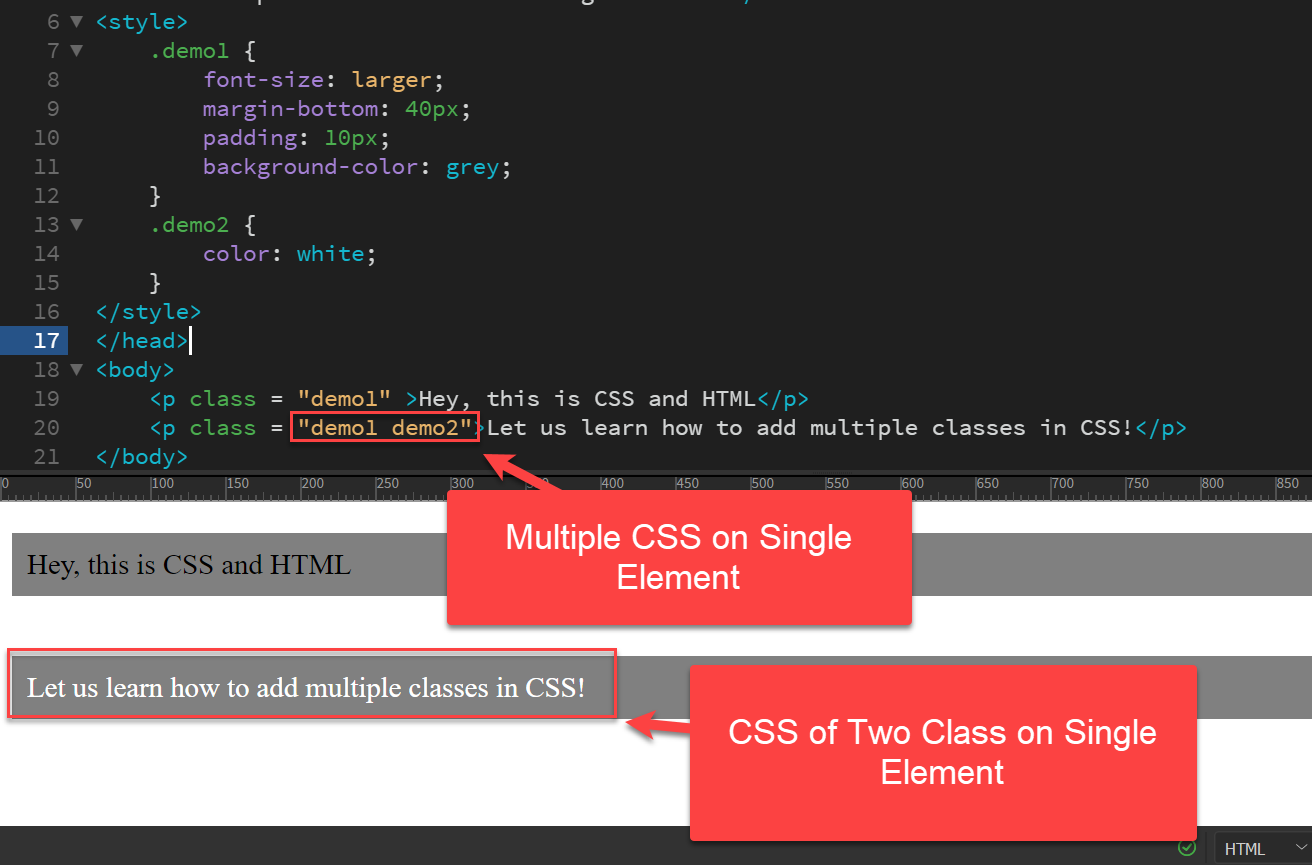
Two or Multiple CSS Classes on a Single HTML Element
Isotopes have the same number of positive particles, called protons, and. Different forms of the same element are called isotopes. Allotropy describes how elements like carbon or oxygen can exist in different structural forms within the same physical.
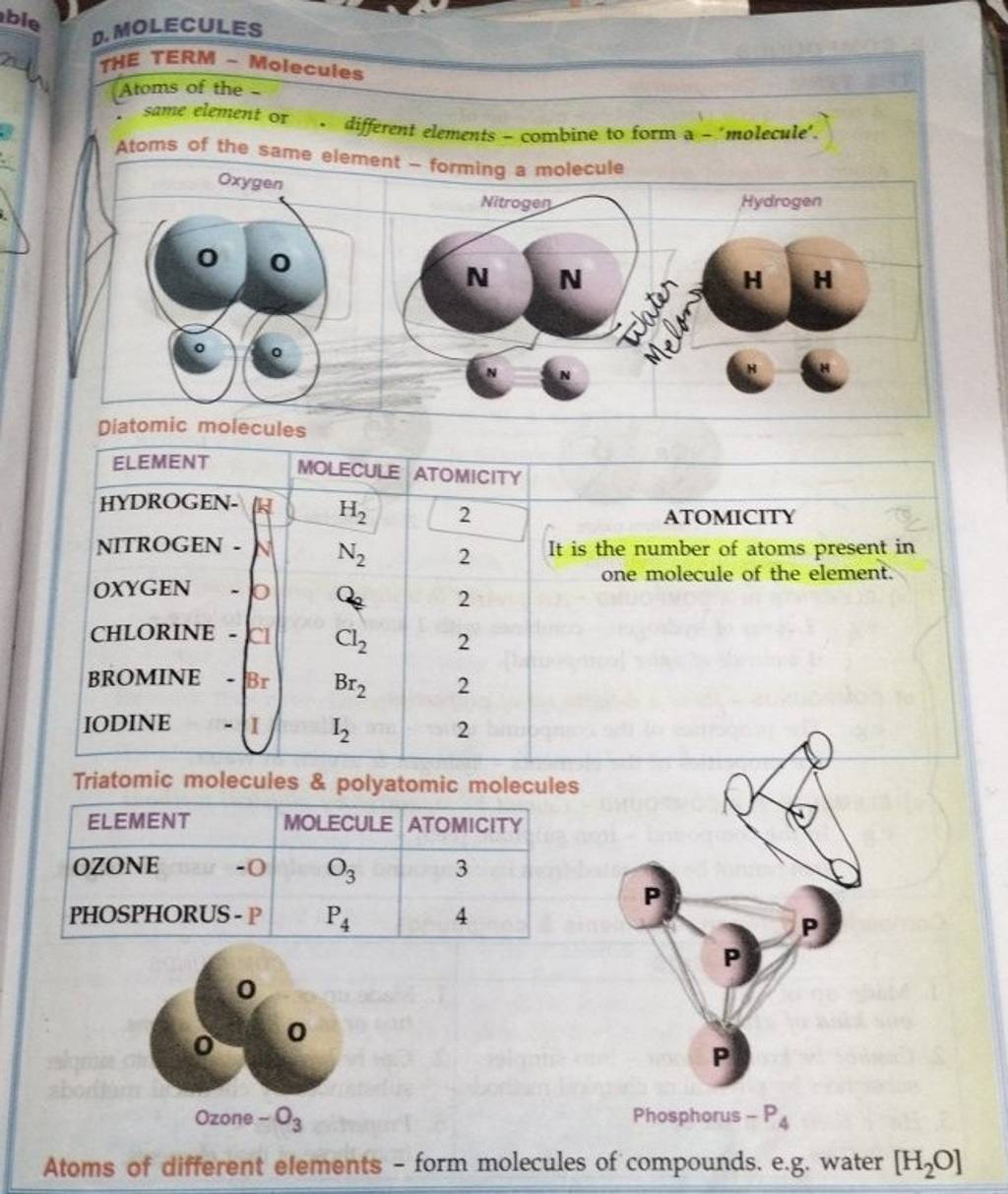
THE TERM MoleculesAtoms of the Same element or. different elements..
Isotopes have the same number of positive particles, called protons, and. Allotropy describes how elements like carbon or oxygen can exist in different structural forms within the same physical. Different forms of the same element are called isotopes.
Works of Abstract Art with Different Forms. Vector Illustration Design
Allotropy describes how elements like carbon or oxygen can exist in different structural forms within the same physical. Different forms of the same element are called isotopes. Isotopes have the same number of positive particles, called protons, and.
terminology Can an element be a single atom or a molecule made up of
Isotopes have the same number of positive particles, called protons, and. Allotropy describes how elements like carbon or oxygen can exist in different structural forms within the same physical. Different forms of the same element are called isotopes.
Worksheet Templates Isotopes Of The Same Element Have Different
Isotopes have the same number of positive particles, called protons, and. Allotropy describes how elements like carbon or oxygen can exist in different structural forms within the same physical. Different forms of the same element are called isotopes.
Are Two Atoms of the Same Element Identical?
Different forms of the same element are called isotopes. Isotopes have the same number of positive particles, called protons, and. Allotropy describes how elements like carbon or oxygen can exist in different structural forms within the same physical.
Atoms of the Same Element Can Have Different Properties
Different forms of the same element are called isotopes. Isotopes have the same number of positive particles, called protons, and. Allotropy describes how elements like carbon or oxygen can exist in different structural forms within the same physical.
3. All the atoms of a same element have identical mass and identical chem..
Allotropy describes how elements like carbon or oxygen can exist in different structural forms within the same physical. Different forms of the same element are called isotopes. Isotopes have the same number of positive particles, called protons, and.
Isotopes Have The Same Number Of Positive Particles, Called Protons, And.
Allotropy describes how elements like carbon or oxygen can exist in different structural forms within the same physical. Different forms of the same element are called isotopes.