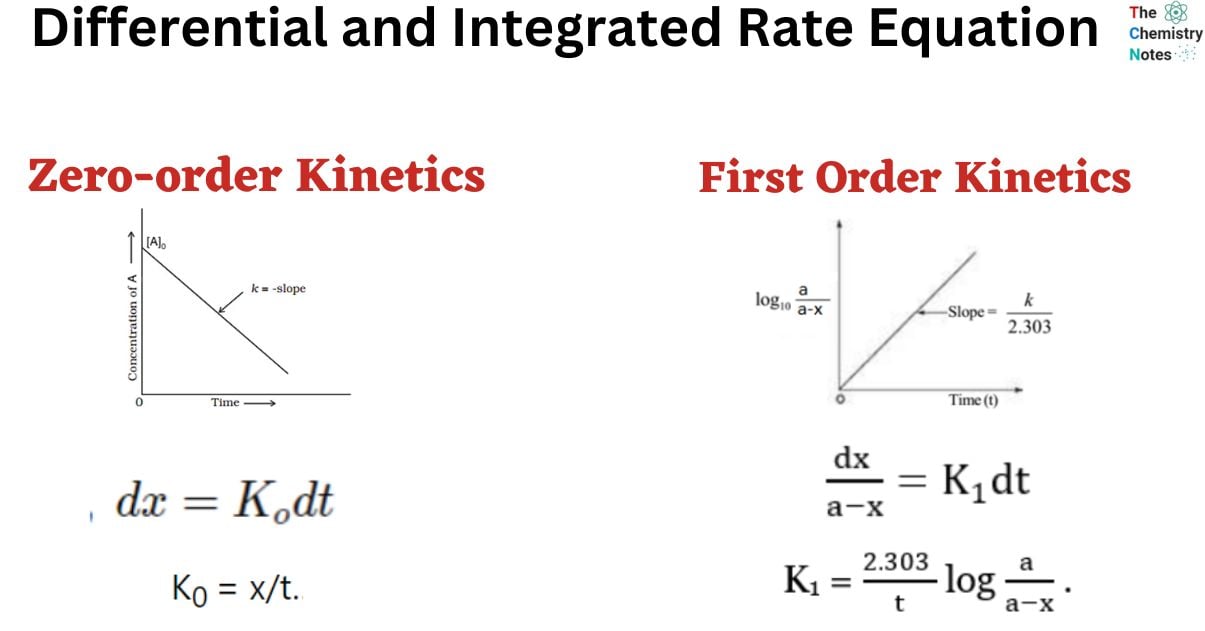
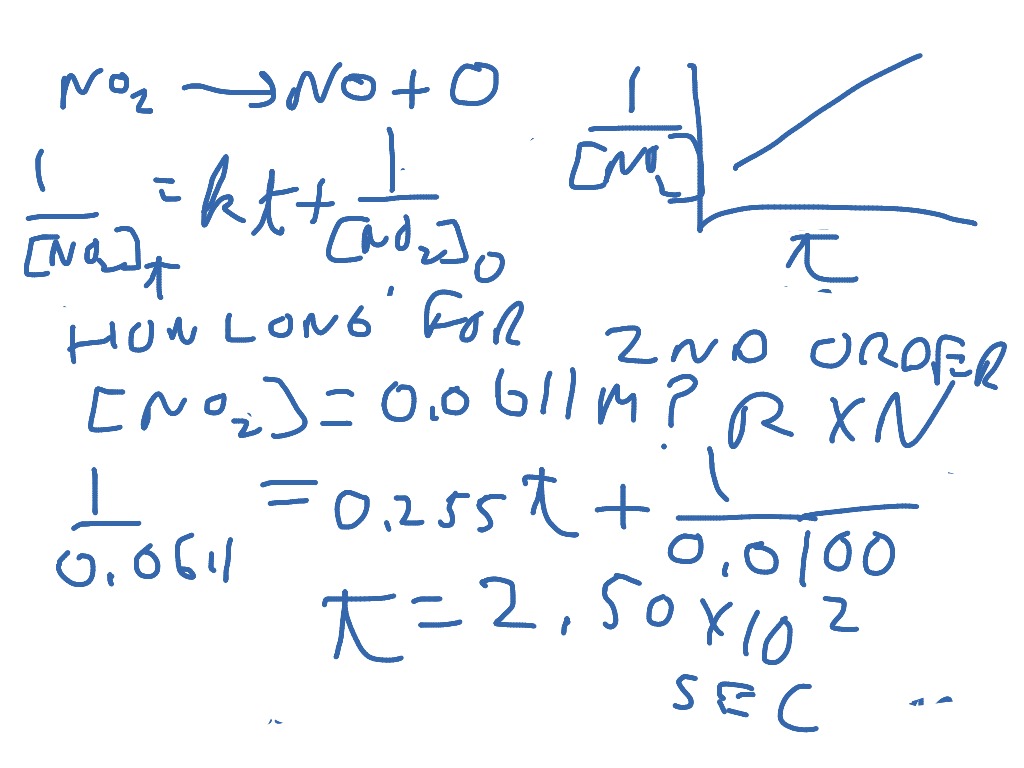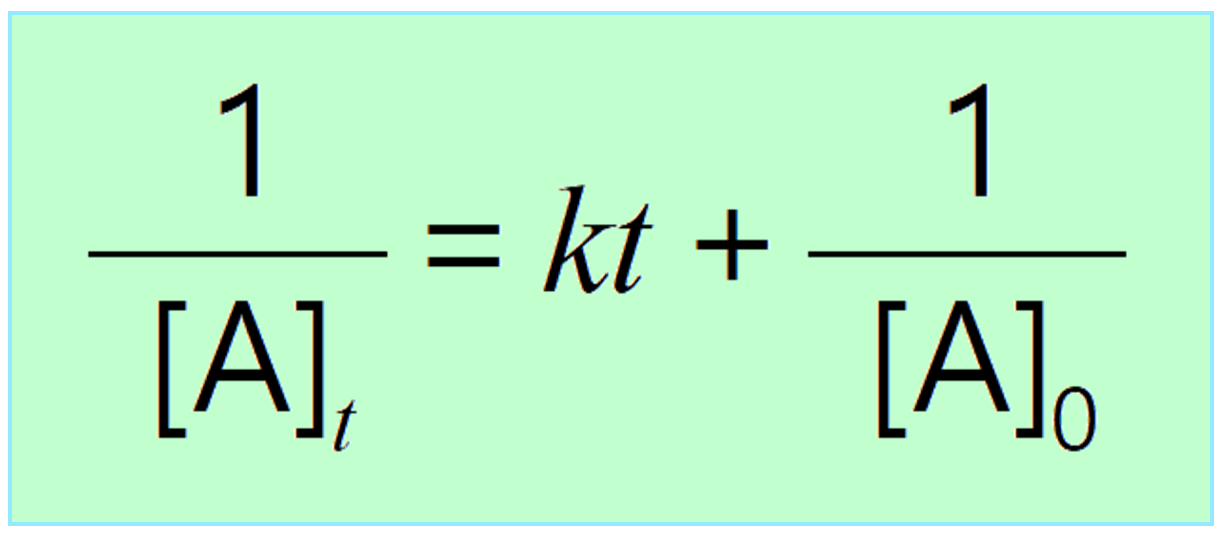Differential Vs Integrated Rate Law - Differential rate law and integrated rate law are two mathematical expressions used to describe the. Differential rate law = expresses how the reaction rate depends on the concentration of reactant (s). Either the differential rate law or the integrated rate law can be used to determine the. What is the main difference between differential rate laws and integrated rate laws?. The main difference between differential rate law and integrated rate law is that. As it turns out, rate laws can actually be written using two different, but related, perspectives. Using calculus, the differential rate law for a chemical reaction can be integrated with.
Differential rate law = expresses how the reaction rate depends on the concentration of reactant (s). Using calculus, the differential rate law for a chemical reaction can be integrated with. The main difference between differential rate law and integrated rate law is that. As it turns out, rate laws can actually be written using two different, but related, perspectives. Either the differential rate law or the integrated rate law can be used to determine the. What is the main difference between differential rate laws and integrated rate laws?. Differential rate law and integrated rate law are two mathematical expressions used to describe the.
The main difference between differential rate law and integrated rate law is that. Using calculus, the differential rate law for a chemical reaction can be integrated with. Differential rate law = expresses how the reaction rate depends on the concentration of reactant (s). As it turns out, rate laws can actually be written using two different, but related, perspectives. Either the differential rate law or the integrated rate law can be used to determine the. What is the main difference between differential rate laws and integrated rate laws?. Differential rate law and integrated rate law are two mathematical expressions used to describe the.
Rate Law and Integrated Rate Law Diagram Quizlet
Using calculus, the differential rate law for a chemical reaction can be integrated with. What is the main difference between differential rate laws and integrated rate laws?. As it turns out, rate laws can actually be written using two different, but related, perspectives. Either the differential rate law or the integrated rate law can be used to determine the. Differential.
ShowMe integrated rate law
Differential rate law and integrated rate law are two mathematical expressions used to describe the. Either the differential rate law or the integrated rate law can be used to determine the. As it turns out, rate laws can actually be written using two different, but related, perspectives. Using calculus, the differential rate law for a chemical reaction can be integrated.
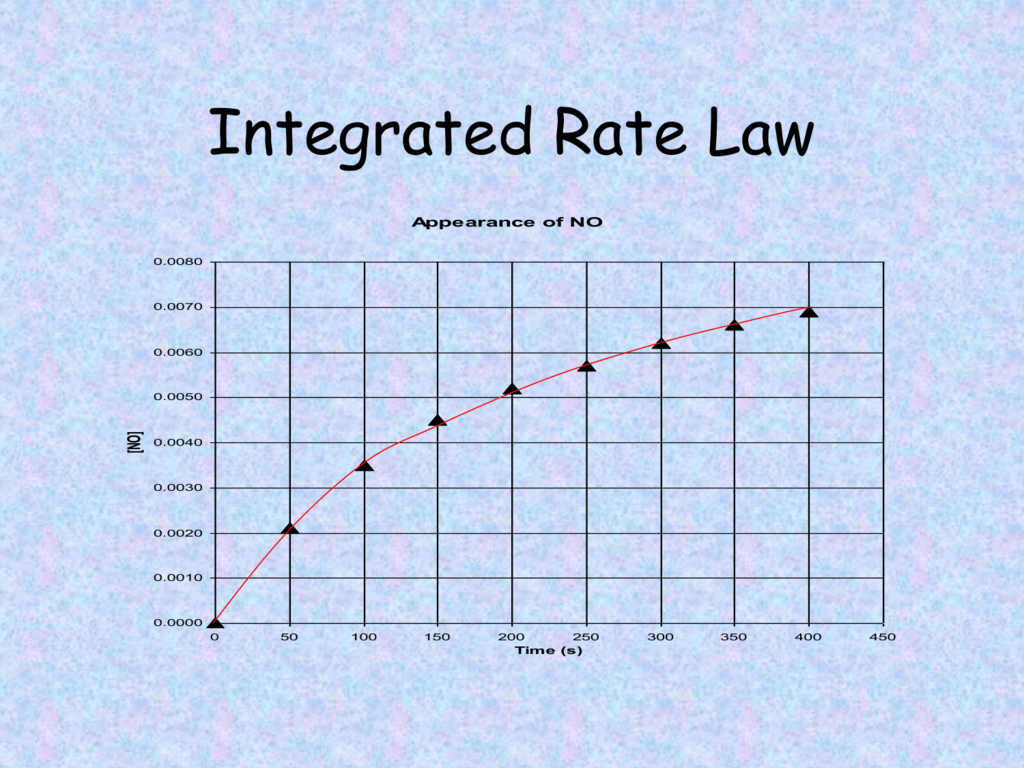
Integrated Rate Law
The main difference between differential rate law and integrated rate law is that. Either the differential rate law or the integrated rate law can be used to determine the. What is the main difference between differential rate laws and integrated rate laws?. Differential rate law = expresses how the reaction rate depends on the concentration of reactant (s). Using calculus,.
Integrated Rate Law Chemistry Steps
What is the main difference between differential rate laws and integrated rate laws?. Using calculus, the differential rate law for a chemical reaction can be integrated with. The main difference between differential rate law and integrated rate law is that. Either the differential rate law or the integrated rate law can be used to determine the. As it turns out,.
Integrated rate law graphs Chemistry Stack Exchange
The main difference between differential rate law and integrated rate law is that. Either the differential rate law or the integrated rate law can be used to determine the. Differential rate law and integrated rate law are two mathematical expressions used to describe the. Differential rate law = expresses how the reaction rate depends on the concentration of reactant (s)..
Integrated Rate Law Chemistry Steps
Either the differential rate law or the integrated rate law can be used to determine the. Differential rate law = expresses how the reaction rate depends on the concentration of reactant (s). The main difference between differential rate law and integrated rate law is that. Differential rate law and integrated rate law are two mathematical expressions used to describe the..
Differential and Integrated Rate Equation
Using calculus, the differential rate law for a chemical reaction can be integrated with. As it turns out, rate laws can actually be written using two different, but related, perspectives. The main difference between differential rate law and integrated rate law is that. Either the differential rate law or the integrated rate law can be used to determine the. What.
Solved From the differential rate law for a secondorder
As it turns out, rate laws can actually be written using two different, but related, perspectives. Differential rate law = expresses how the reaction rate depends on the concentration of reactant (s). Using calculus, the differential rate law for a chemical reaction can be integrated with. Differential rate law and integrated rate law are two mathematical expressions used to describe.
24++ Derive The Integrated Rate Law For First Order Reaction Marcodd
Using calculus, the differential rate law for a chemical reaction can be integrated with. The main difference between differential rate law and integrated rate law is that. Differential rate law = expresses how the reaction rate depends on the concentration of reactant (s). Either the differential rate law or the integrated rate law can be used to determine the. What.
Second Order Integrated Rate Law Equation OntoBel
Differential rate law = expresses how the reaction rate depends on the concentration of reactant (s). What is the main difference between differential rate laws and integrated rate laws?. Either the differential rate law or the integrated rate law can be used to determine the. Differential rate law and integrated rate law are two mathematical expressions used to describe the..
Differential Rate Law And Integrated Rate Law Are Two Mathematical Expressions Used To Describe The.
The main difference between differential rate law and integrated rate law is that. Using calculus, the differential rate law for a chemical reaction can be integrated with. Either the differential rate law or the integrated rate law can be used to determine the. As it turns out, rate laws can actually be written using two different, but related, perspectives.
What Is The Main Difference Between Differential Rate Laws And Integrated Rate Laws?.
Differential rate law = expresses how the reaction rate depends on the concentration of reactant (s).