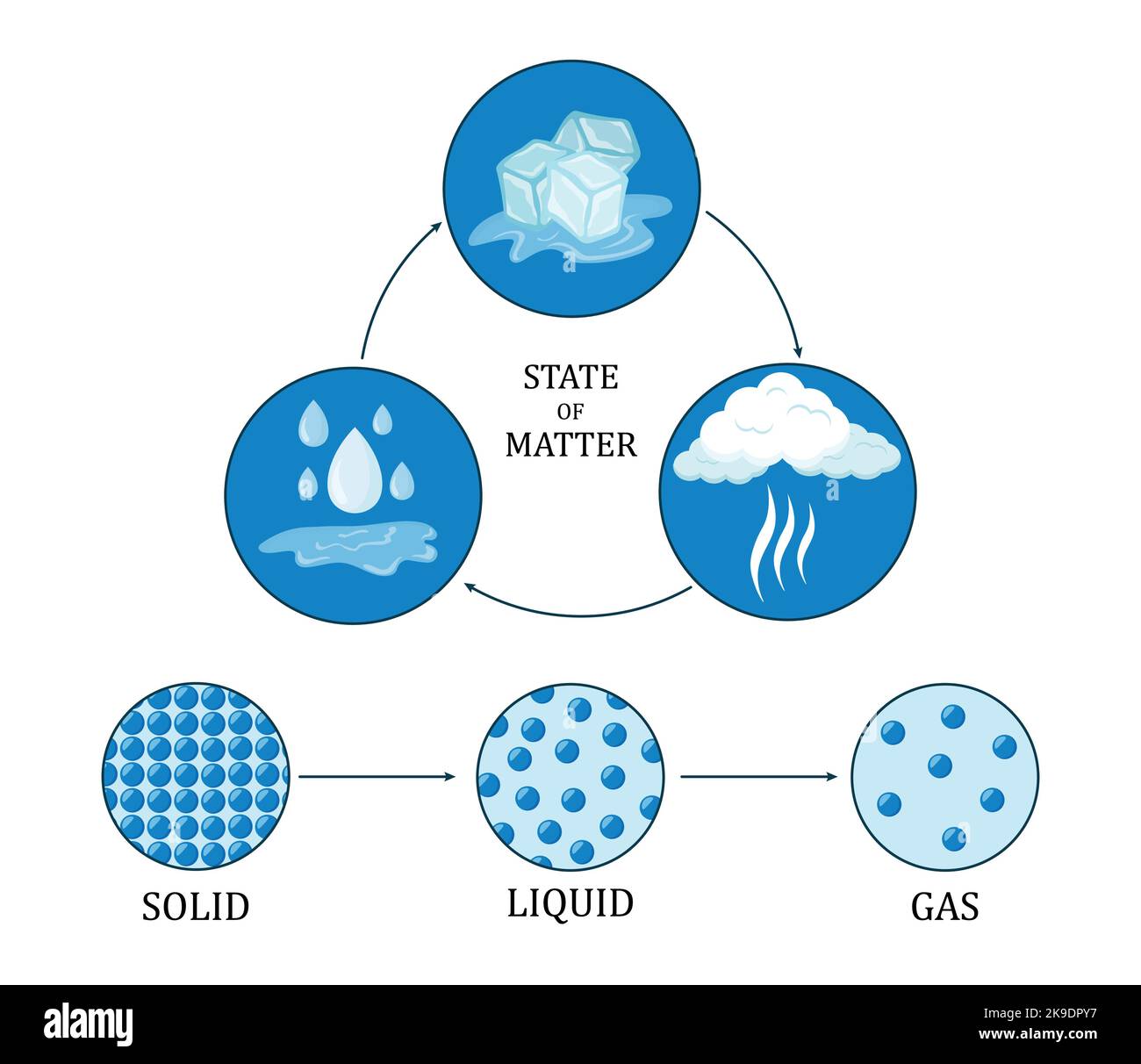
Differentiate Solid Liquid And Gas - Difference between solids, liquids, and gas overview. The particles in solids are tightly packed in an organized arrangement. An object that is rigid, has a low intermolecular space between molecules. Solids are characterized by a fixed shape and volume.
Solids are characterized by a fixed shape and volume. Difference between solids, liquids, and gas overview. An object that is rigid, has a low intermolecular space between molecules. The particles in solids are tightly packed in an organized arrangement.
The particles in solids are tightly packed in an organized arrangement. Solids are characterized by a fixed shape and volume. Difference between solids, liquids, and gas overview. An object that is rigid, has a low intermolecular space between molecules.
Properties of Solid, Liquid, Gases A Comparison
Difference between solids, liquids, and gas overview. An object that is rigid, has a low intermolecular space between molecules. Solids are characterized by a fixed shape and volume. The particles in solids are tightly packed in an organized arrangement.
Gas In Liquid Or Solid at William Derr blog
Solids are characterized by a fixed shape and volume. Difference between solids, liquids, and gas overview. An object that is rigid, has a low intermolecular space between molecules. The particles in solids are tightly packed in an organized arrangement.
Different States Of Matter Solid, Liquid, Gas Vector, 60 OFF
Solids are characterized by a fixed shape and volume. An object that is rigid, has a low intermolecular space between molecules. The particles in solids are tightly packed in an organized arrangement. Difference between solids, liquids, and gas overview.
What is difference between solid liquid and gas Chemistry Matter
The particles in solids are tightly packed in an organized arrangement. Solids are characterized by a fixed shape and volume. An object that is rigid, has a low intermolecular space between molecules. Difference between solids, liquids, and gas overview.
Solid Liquid Gas Chart
Difference between solids, liquids, and gas overview. The particles in solids are tightly packed in an organized arrangement. An object that is rigid, has a low intermolecular space between molecules. Solids are characterized by a fixed shape and volume.
SOLUTION Solid liquid gas illustration and example Studypool
Solids are characterized by a fixed shape and volume. An object that is rigid, has a low intermolecular space between molecules. Difference between solids, liquids, and gas overview. The particles in solids are tightly packed in an organized arrangement.
solid liquid and gas posters Made By Teachers
The particles in solids are tightly packed in an organized arrangement. Difference between solids, liquids, and gas overview. An object that is rigid, has a low intermolecular space between molecules. Solids are characterized by a fixed shape and volume.
states of matter, solid, liquid and gas in 2024 Solid liquid gas
The particles in solids are tightly packed in an organized arrangement. An object that is rigid, has a low intermolecular space between molecules. Solids are characterized by a fixed shape and volume. Difference between solids, liquids, and gas overview.
Solid Liquid Gas Project
Solids are characterized by a fixed shape and volume. The particles in solids are tightly packed in an organized arrangement. An object that is rigid, has a low intermolecular space between molecules. Difference between solids, liquids, and gas overview.
States of Matter As Solid, Liquid and Gas Physical Types Outline
The particles in solids are tightly packed in an organized arrangement. Solids are characterized by a fixed shape and volume. An object that is rigid, has a low intermolecular space between molecules. Difference between solids, liquids, and gas overview.
Difference Between Solids, Liquids, And Gas Overview.
An object that is rigid, has a low intermolecular space between molecules. The particles in solids are tightly packed in an organized arrangement. Solids are characterized by a fixed shape and volume.