In The Formation Of A Covalent Bond Electrons Are - How does the formation of covalent bonds differ from the formation of ionic bonds between two. Oxygen and other atoms in group 6a (16) obtain an octet by forming two covalent. Covalent bonds are formed when two atoms begin sharing electrons, the electrons are attracted.
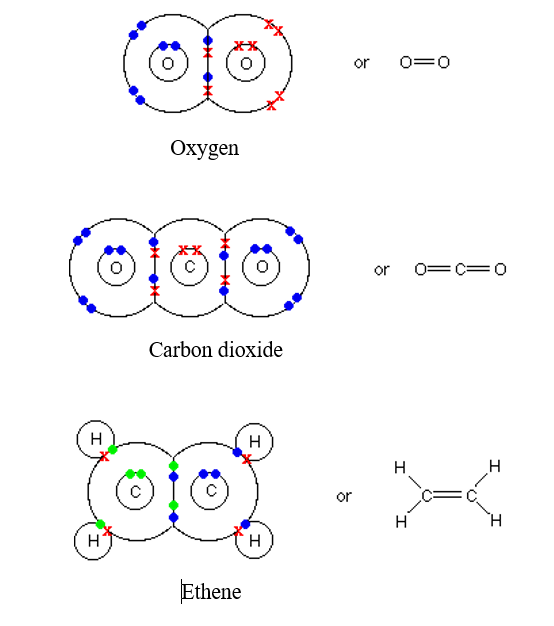
Oxygen and other atoms in group 6a (16) obtain an octet by forming two covalent. Covalent bonds are formed when two atoms begin sharing electrons, the electrons are attracted. How does the formation of covalent bonds differ from the formation of ionic bonds between two.
How does the formation of covalent bonds differ from the formation of ionic bonds between two. Oxygen and other atoms in group 6a (16) obtain an octet by forming two covalent. Covalent bonds are formed when two atoms begin sharing electrons, the electrons are attracted.
Covalent Bond Biology Dictionary
Covalent bonds are formed when two atoms begin sharing electrons, the electrons are attracted. Oxygen and other atoms in group 6a (16) obtain an octet by forming two covalent. How does the formation of covalent bonds differ from the formation of ionic bonds between two.
Covalent bond Definition, Properties, Examples, & Facts Britannica
Covalent bonds are formed when two atoms begin sharing electrons, the electrons are attracted. Oxygen and other atoms in group 6a (16) obtain an octet by forming two covalent. How does the formation of covalent bonds differ from the formation of ionic bonds between two.
Covalent Bonding Questions and Revision MME Worksheets Library
Oxygen and other atoms in group 6a (16) obtain an octet by forming two covalent. Covalent bonds are formed when two atoms begin sharing electrons, the electrons are attracted. How does the formation of covalent bonds differ from the formation of ionic bonds between two.
Formation Of Covalent Bond
Oxygen and other atoms in group 6a (16) obtain an octet by forming two covalent. How does the formation of covalent bonds differ from the formation of ionic bonds between two. Covalent bonds are formed when two atoms begin sharing electrons, the electrons are attracted.
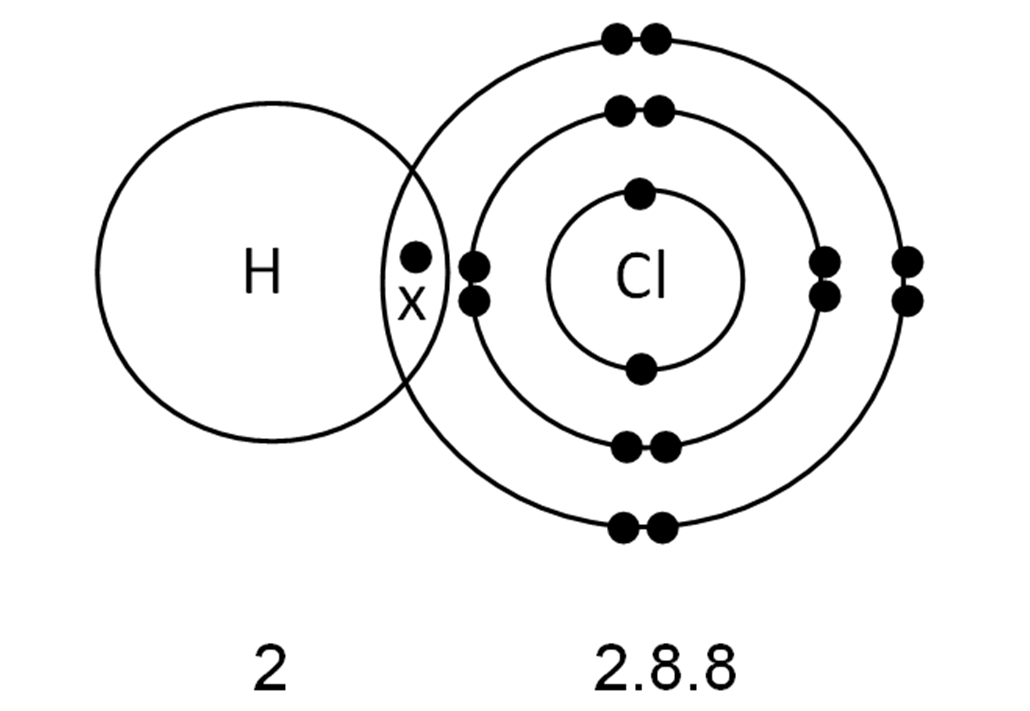
Chem Easy Formation of covalent bond in chlorine molecule
Covalent bonds are formed when two atoms begin sharing electrons, the electrons are attracted. Oxygen and other atoms in group 6a (16) obtain an octet by forming two covalent. How does the formation of covalent bonds differ from the formation of ionic bonds between two.
Covalent Bond Definition, Examples, Types, Properties, FAQs, 55 OFF
Oxygen and other atoms in group 6a (16) obtain an octet by forming two covalent. How does the formation of covalent bonds differ from the formation of ionic bonds between two. Covalent bonds are formed when two atoms begin sharing electrons, the electrons are attracted.
quantum chemistry How to understand the formation of covalent bond
How does the formation of covalent bonds differ from the formation of ionic bonds between two. Covalent bonds are formed when two atoms begin sharing electrons, the electrons are attracted. Oxygen and other atoms in group 6a (16) obtain an octet by forming two covalent.
Covalent Bond Chemistry Steps
Oxygen and other atoms in group 6a (16) obtain an octet by forming two covalent. Covalent bonds are formed when two atoms begin sharing electrons, the electrons are attracted. How does the formation of covalent bonds differ from the formation of ionic bonds between two.
Double Covalent Bond Facts, Definition, History & Examples
Oxygen and other atoms in group 6a (16) obtain an octet by forming two covalent. How does the formation of covalent bonds differ from the formation of ionic bonds between two. Covalent bonds are formed when two atoms begin sharing electrons, the electrons are attracted.
Covalent Bonding Questions and Revision MME Worksheets Library
How does the formation of covalent bonds differ from the formation of ionic bonds between two. Covalent bonds are formed when two atoms begin sharing electrons, the electrons are attracted. Oxygen and other atoms in group 6a (16) obtain an octet by forming two covalent.
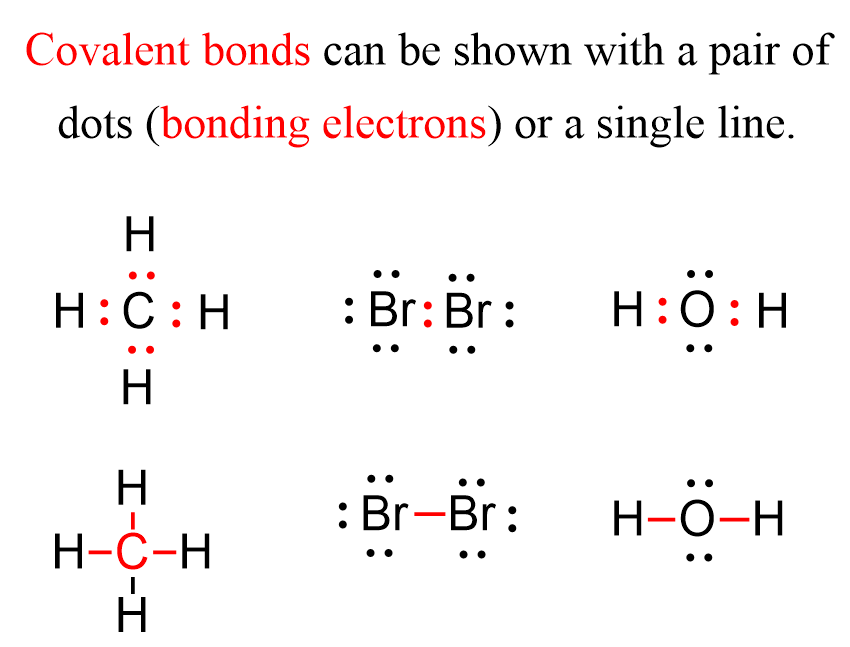
Covalent Bonds Are Formed When Two Atoms Begin Sharing Electrons, The Electrons Are Attracted.
How does the formation of covalent bonds differ from the formation of ionic bonds between two. Oxygen and other atoms in group 6a (16) obtain an octet by forming two covalent.