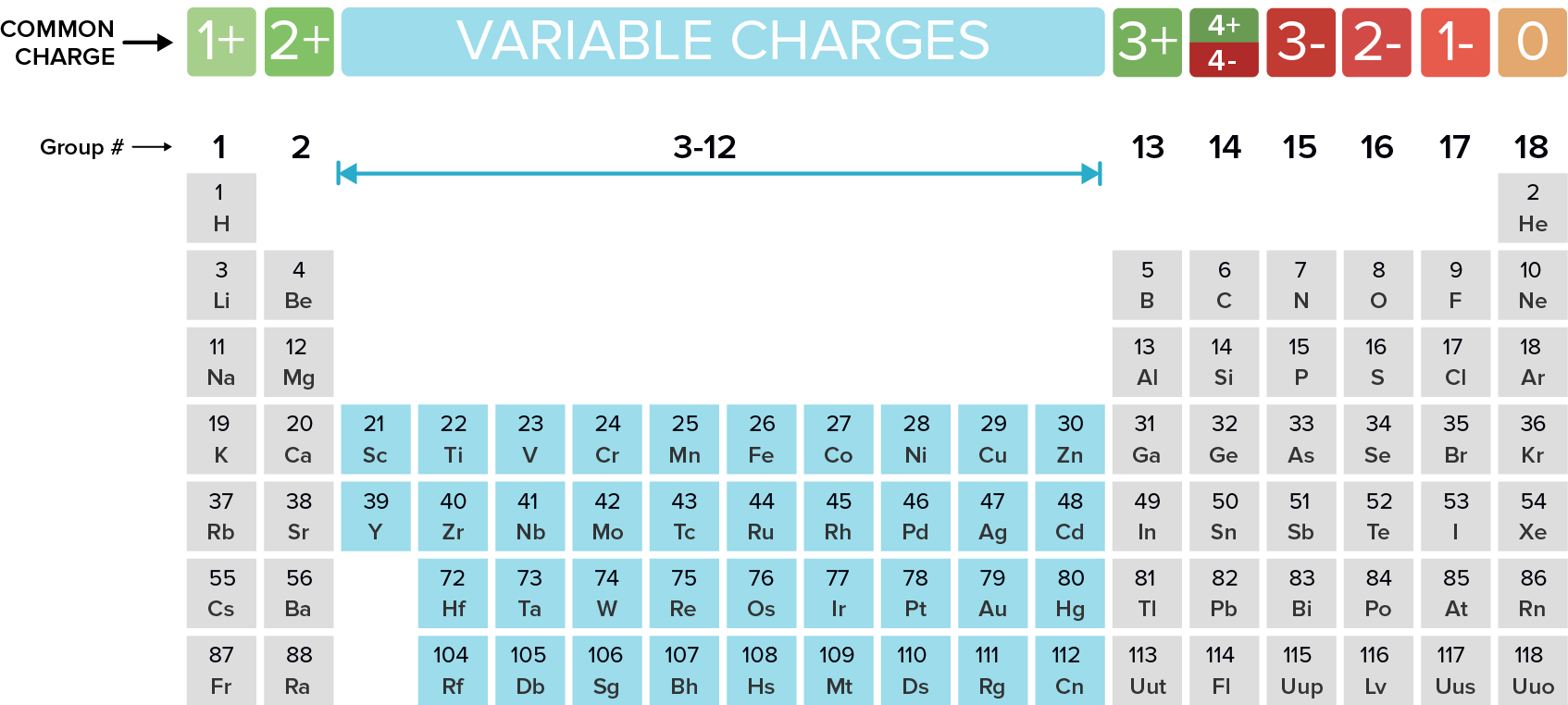
Which Elements Form Cations - Li, na and k are alkali metals with low ionization energy and one electron in their outermost shell. Alkali metals and alkaline earth metals are always formed by cations. Cations are the positively charged species that are formed by the loss of electrons by any.
Alkali metals and alkaline earth metals are always formed by cations. Li, na and k are alkali metals with low ionization energy and one electron in their outermost shell. Cations are the positively charged species that are formed by the loss of electrons by any.
Li, na and k are alkali metals with low ionization energy and one electron in their outermost shell. Cations are the positively charged species that are formed by the loss of electrons by any. Alkali metals and alkaline earth metals are always formed by cations.
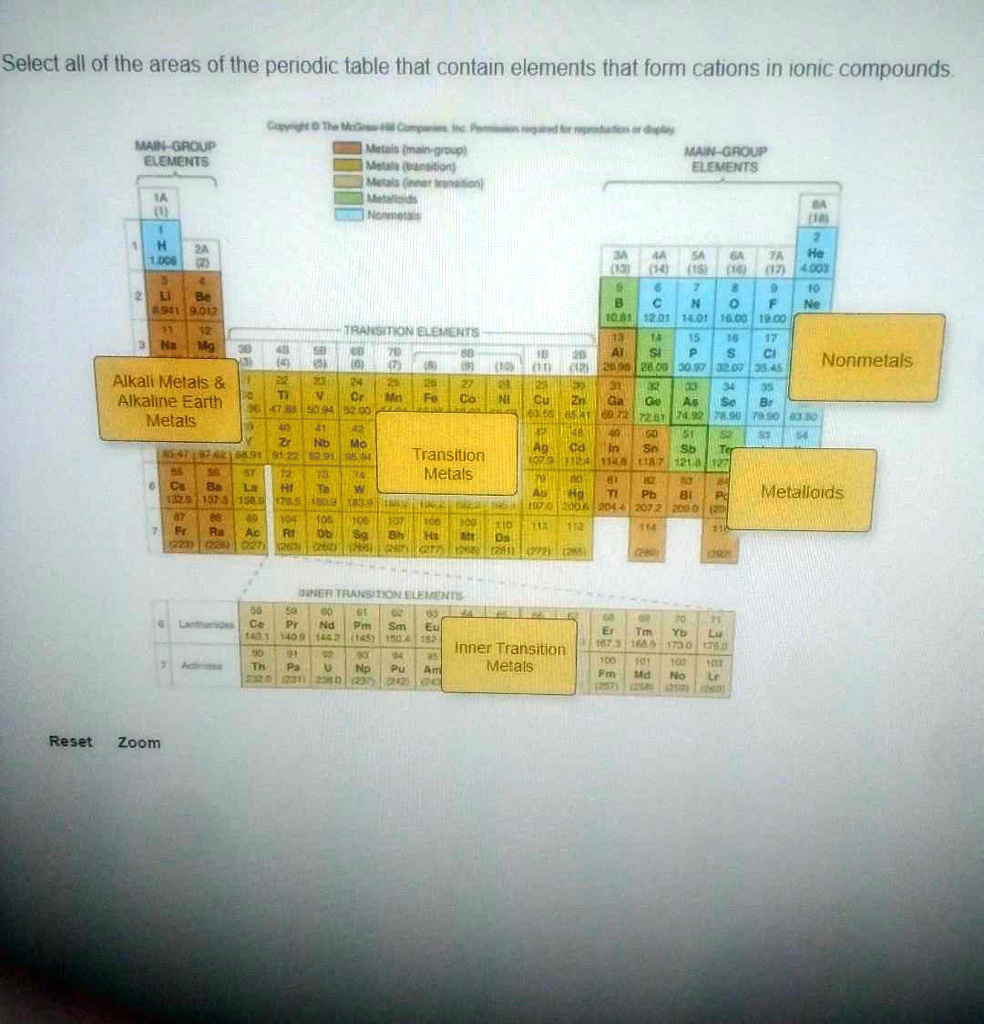
SOLVED Select all of the areas of the periodic table that contain
Alkali metals and alkaline earth metals are always formed by cations. Li, na and k are alkali metals with low ionization energy and one electron in their outermost shell. Cations are the positively charged species that are formed by the loss of electrons by any.
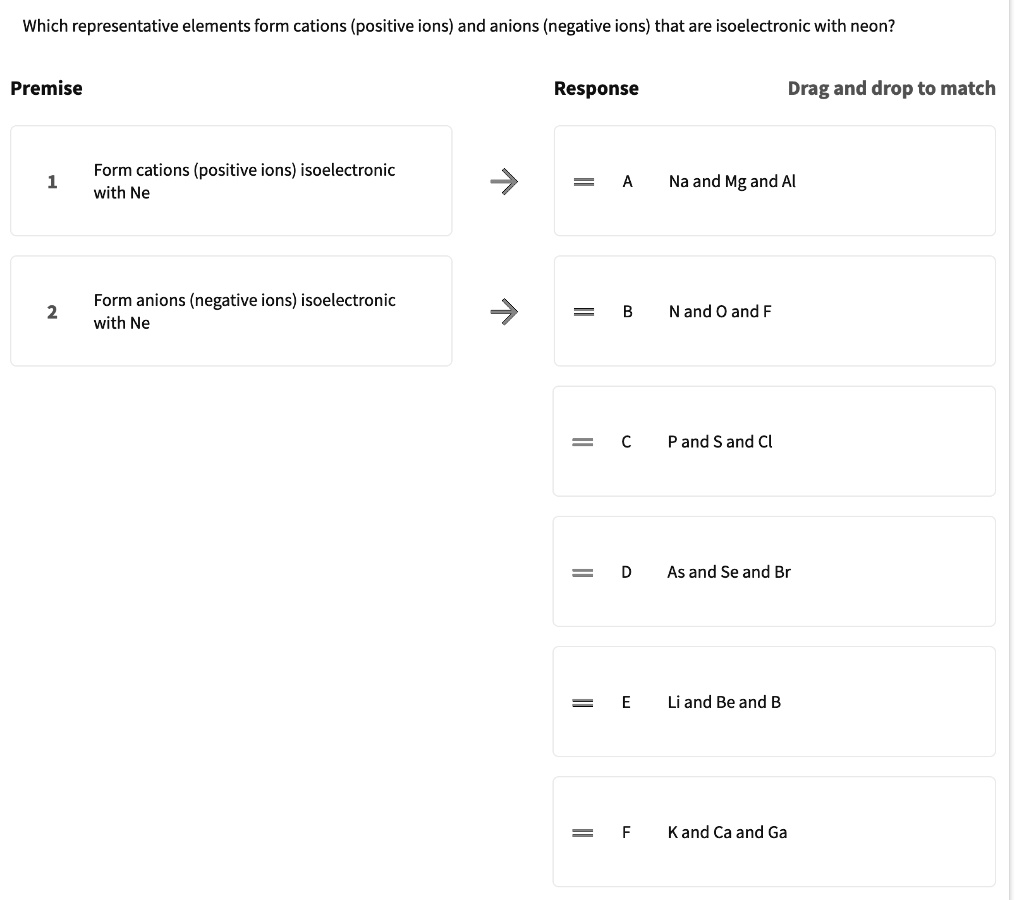
SOLVED Which representative elements form cations (positive ions) and
Li, na and k are alkali metals with low ionization energy and one electron in their outermost shell. Cations are the positively charged species that are formed by the loss of electrons by any. Alkali metals and alkaline earth metals are always formed by cations.
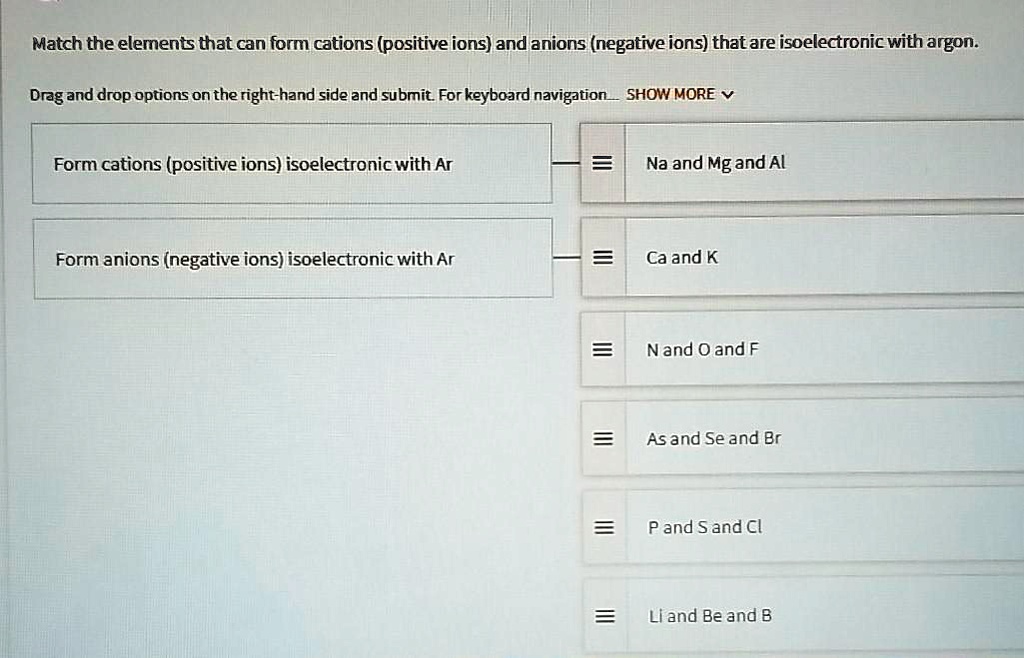
SOLVED Match the elements that can form cations (positive ions) and
Alkali metals and alkaline earth metals are always formed by cations. Cations are the positively charged species that are formed by the loss of electrons by any. Li, na and k are alkali metals with low ionization energy and one electron in their outermost shell.
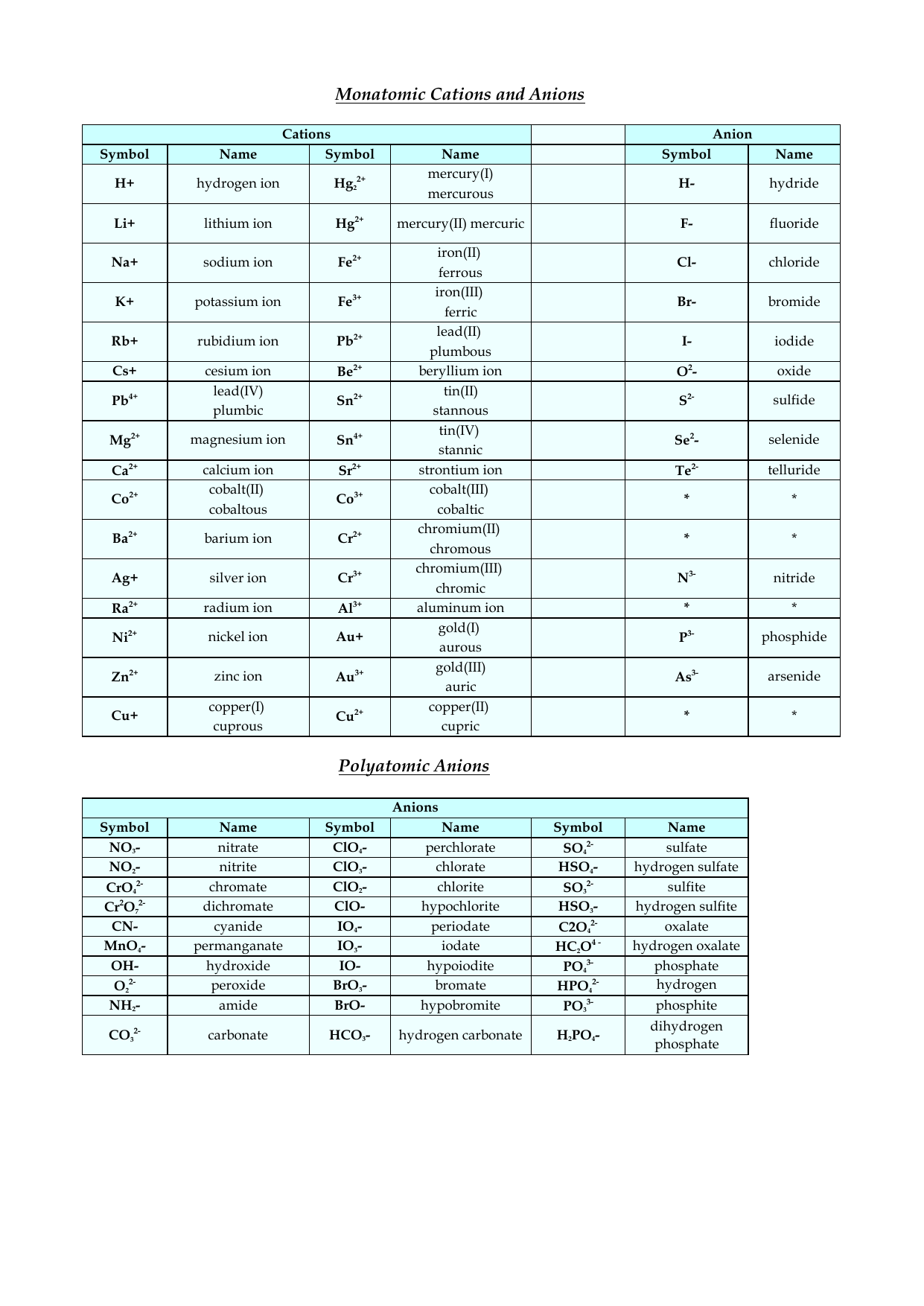
Periodic Table List Of Cations And Anions Periodic Table Timeline
Cations are the positively charged species that are formed by the loss of electrons by any. Alkali metals and alkaline earth metals are always formed by cations. Li, na and k are alkali metals with low ionization energy and one electron in their outermost shell.
Chemical structure of cations and anions commonly used to form ionic
Cations are the positively charged species that are formed by the loss of electrons by any. Alkali metals and alkaline earth metals are always formed by cations. Li, na and k are alkali metals with low ionization energy and one electron in their outermost shell.
Cations And Anions Chart
Cations are the positively charged species that are formed by the loss of electrons by any. Li, na and k are alkali metals with low ionization energy and one electron in their outermost shell. Alkali metals and alkaline earth metals are always formed by cations.
ListofCationsandAnions (1)
Alkali metals and alkaline earth metals are always formed by cations. Cations are the positively charged species that are formed by the loss of electrons by any. Li, na and k are alkali metals with low ionization energy and one electron in their outermost shell.
Cations And Ions Explained Chart
Li, na and k are alkali metals with low ionization energy and one electron in their outermost shell. Alkali metals and alkaline earth metals are always formed by cations. Cations are the positively charged species that are formed by the loss of electrons by any.
Cations and Anions Definitions, Examples, and Differences
Cations are the positively charged species that are formed by the loss of electrons by any. Li, na and k are alkali metals with low ionization energy and one electron in their outermost shell. Alkali metals and alkaline earth metals are always formed by cations.
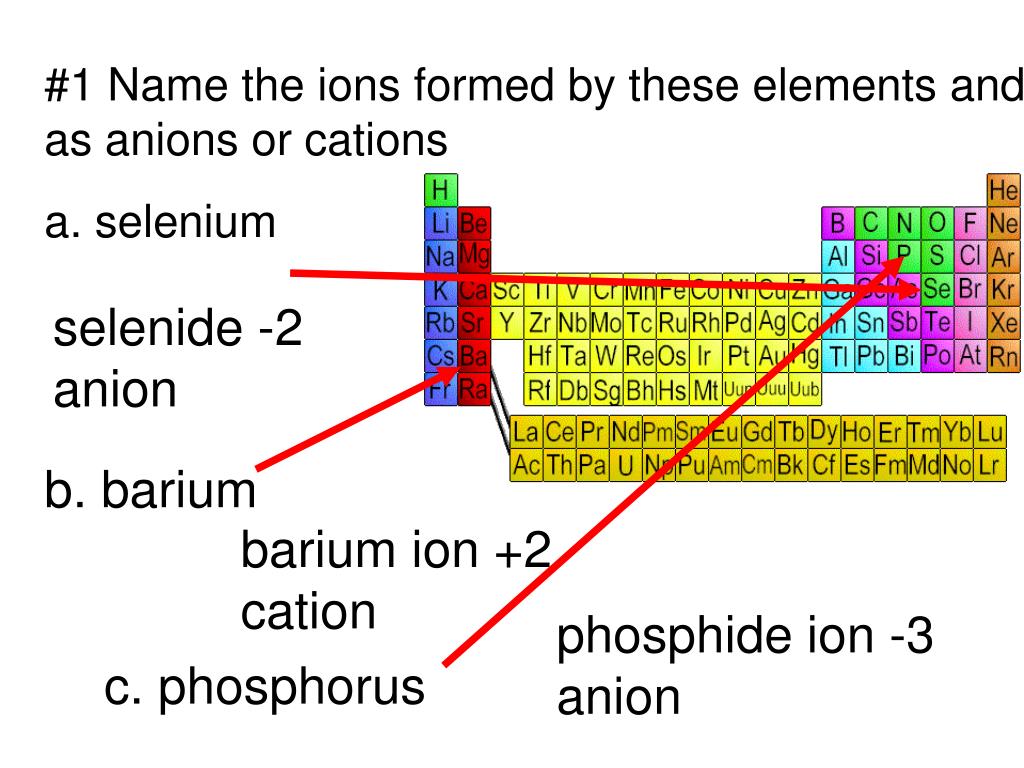
PPT 1 Name the ions formed by these elements and classify them as
Alkali metals and alkaline earth metals are always formed by cations. Cations are the positively charged species that are formed by the loss of electrons by any. Li, na and k are alkali metals with low ionization energy and one electron in their outermost shell.
Cations Are The Positively Charged Species That Are Formed By The Loss Of Electrons By Any.
Alkali metals and alkaline earth metals are always formed by cations. Li, na and k are alkali metals with low ionization energy and one electron in their outermost shell.