Your Informed Consent Form Must Describe - It must contain all the required components of informed consent, as defined in. Is it possible to obtain. The informed consent form must begin with a concise and focused presentation of key. Informed consent is more than merely a signature on a document; What is informed consent and when, why, and how must it be obtained? Under the federal regulations, a pi must obtain “legally effective” informed consent in order to. Learn how to write and use an informed consent form for research studies involving human. Consent for participation in research requires an informed consent process.
Learn how to write and use an informed consent form for research studies involving human. It must contain all the required components of informed consent, as defined in. Consent for participation in research requires an informed consent process. Is it possible to obtain. Under the federal regulations, a pi must obtain “legally effective” informed consent in order to. The informed consent form must begin with a concise and focused presentation of key. What is informed consent and when, why, and how must it be obtained? Informed consent is more than merely a signature on a document;
What is informed consent and when, why, and how must it be obtained? It must contain all the required components of informed consent, as defined in. Consent for participation in research requires an informed consent process. Learn how to write and use an informed consent form for research studies involving human. The informed consent form must begin with a concise and focused presentation of key. Under the federal regulations, a pi must obtain “legally effective” informed consent in order to. Is it possible to obtain. Informed consent is more than merely a signature on a document;
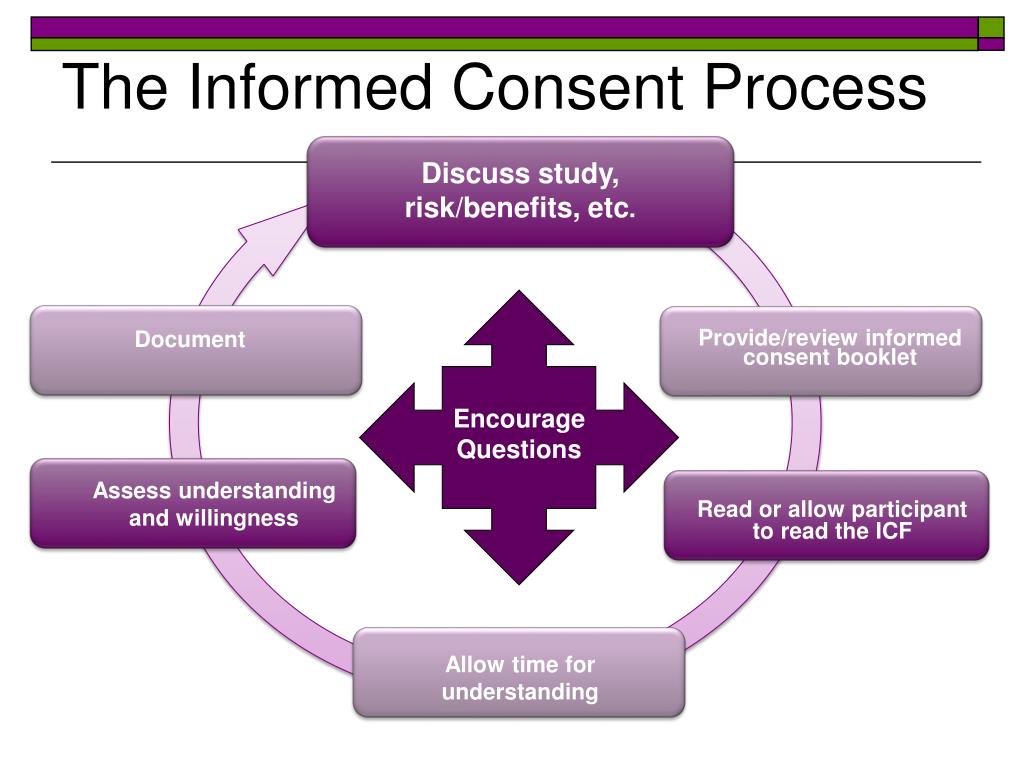
Graphical model of obtaining informed consent form process Download
What is informed consent and when, why, and how must it be obtained? Informed consent is more than merely a signature on a document; The informed consent form must begin with a concise and focused presentation of key. Consent for participation in research requires an informed consent process. Under the federal regulations, a pi must obtain “legally effective” informed consent.
Informed Consent Form Template
Under the federal regulations, a pi must obtain “legally effective” informed consent in order to. Consent for participation in research requires an informed consent process. Learn how to write and use an informed consent form for research studies involving human. Is it possible to obtain. Informed consent is more than merely a signature on a document;
Your Informed Consent Form Must Describe Consent Form Form example
It must contain all the required components of informed consent, as defined in. Is it possible to obtain. Consent for participation in research requires an informed consent process. Informed consent is more than merely a signature on a document; Under the federal regulations, a pi must obtain “legally effective” informed consent in order to.
PPT Informed Consent PowerPoint Presentation, free download ID3519482
It must contain all the required components of informed consent, as defined in. Consent for participation in research requires an informed consent process. Informed consent is more than merely a signature on a document; What is informed consent and when, why, and how must it be obtained? Learn how to write and use an informed consent form for research studies.
Informed consent is a patient right Asante News Site
Is it possible to obtain. What is informed consent and when, why, and how must it be obtained? Under the federal regulations, a pi must obtain “legally effective” informed consent in order to. It must contain all the required components of informed consent, as defined in. Consent for participation in research requires an informed consent process.
Your Informed Consent Form Must Describe Consent Form Form example
Is it possible to obtain. What is informed consent and when, why, and how must it be obtained? It must contain all the required components of informed consent, as defined in. The informed consent form must begin with a concise and focused presentation of key. Learn how to write and use an informed consent form for research studies involving human.
Informed consent form PPT
It must contain all the required components of informed consent, as defined in. Under the federal regulations, a pi must obtain “legally effective” informed consent in order to. Consent for participation in research requires an informed consent process. Informed consent is more than merely a signature on a document; What is informed consent and when, why, and how must it.
Ined S 20182025 Form Fill Out and Sign Printable PDF Template
It must contain all the required components of informed consent, as defined in. Informed consent is more than merely a signature on a document; What is informed consent and when, why, and how must it be obtained? Under the federal regulations, a pi must obtain “legally effective” informed consent in order to. Consent for participation in research requires an informed.
Your Informed Consent Form Must Describe
The informed consent form must begin with a concise and focused presentation of key. It must contain all the required components of informed consent, as defined in. Consent for participation in research requires an informed consent process. Learn how to write and use an informed consent form for research studies involving human. Informed consent is more than merely a signature.
SAMPLE INFORMED CONSENT FORM
Under the federal regulations, a pi must obtain “legally effective” informed consent in order to. Is it possible to obtain. The informed consent form must begin with a concise and focused presentation of key. Learn how to write and use an informed consent form for research studies involving human. Informed consent is more than merely a signature on a document;
It Must Contain All The Required Components Of Informed Consent, As Defined In.
Informed consent is more than merely a signature on a document; Is it possible to obtain. Under the federal regulations, a pi must obtain “legally effective” informed consent in order to. Learn how to write and use an informed consent form for research studies involving human.
What Is Informed Consent And When, Why, And How Must It Be Obtained?
Consent for participation in research requires an informed consent process. The informed consent form must begin with a concise and focused presentation of key.