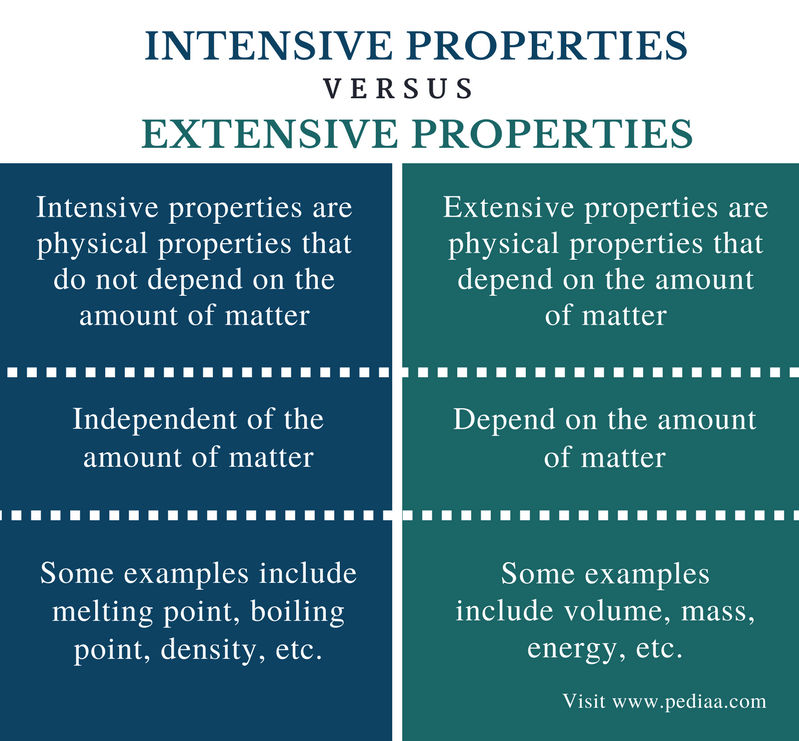
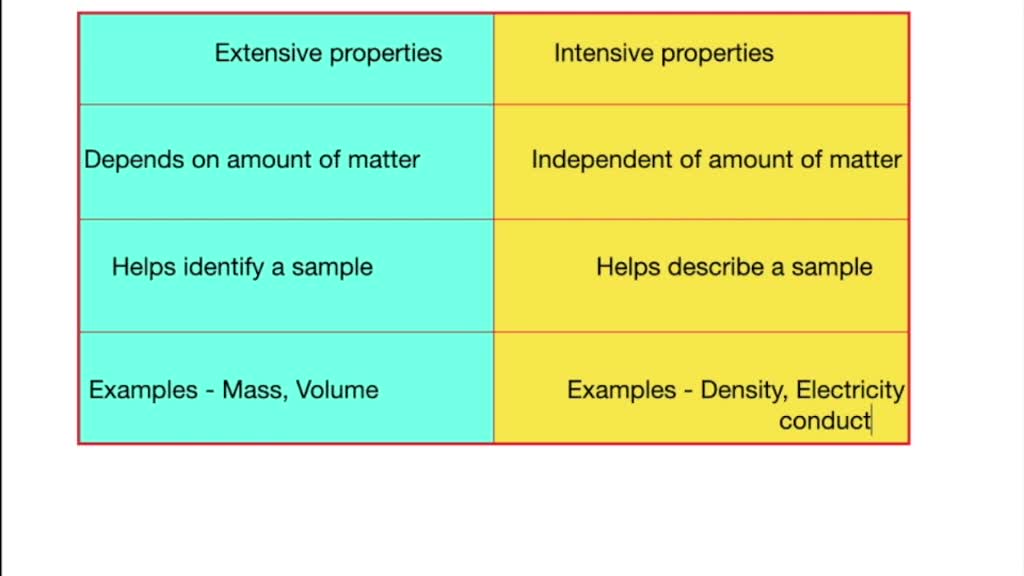
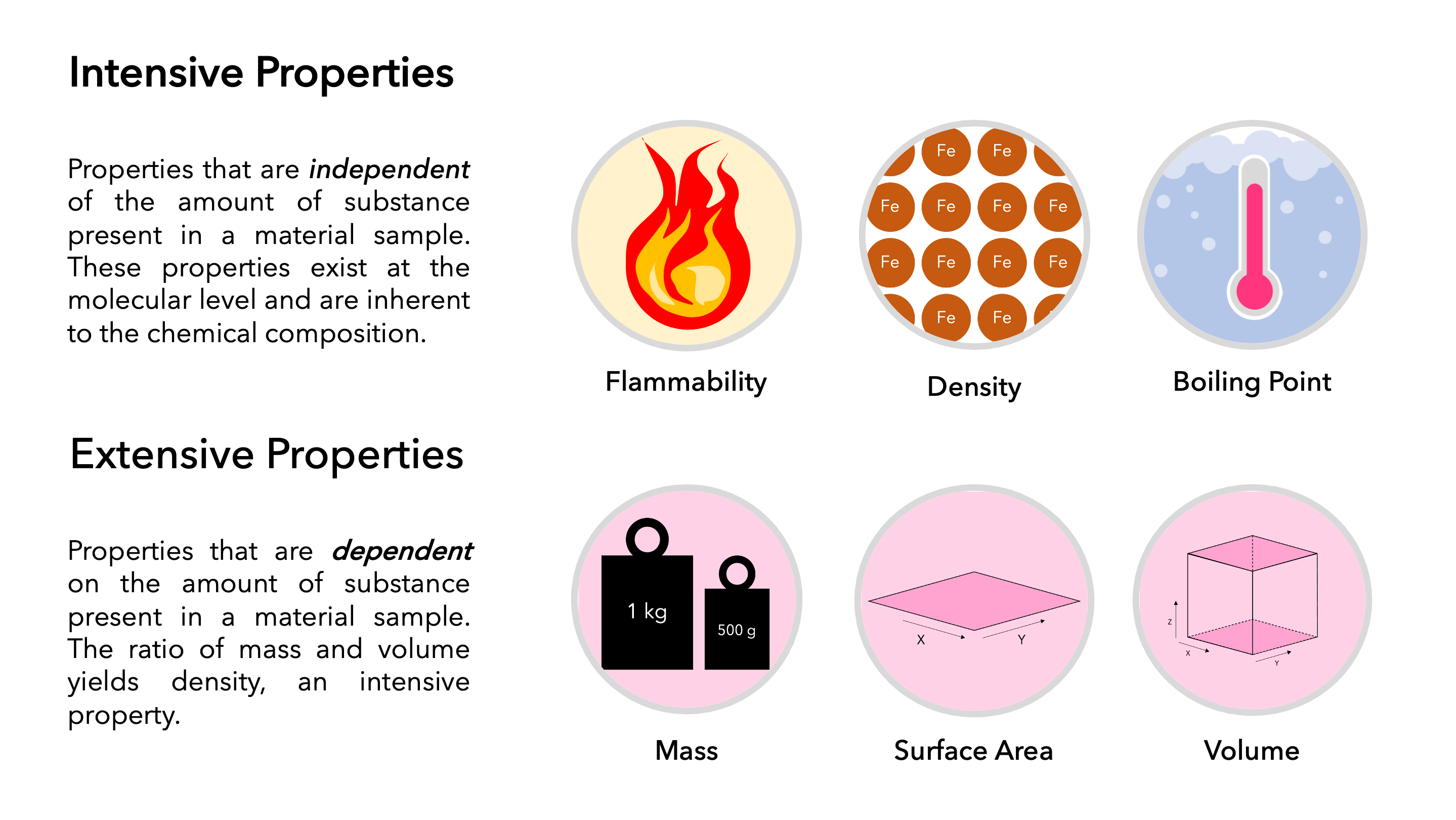
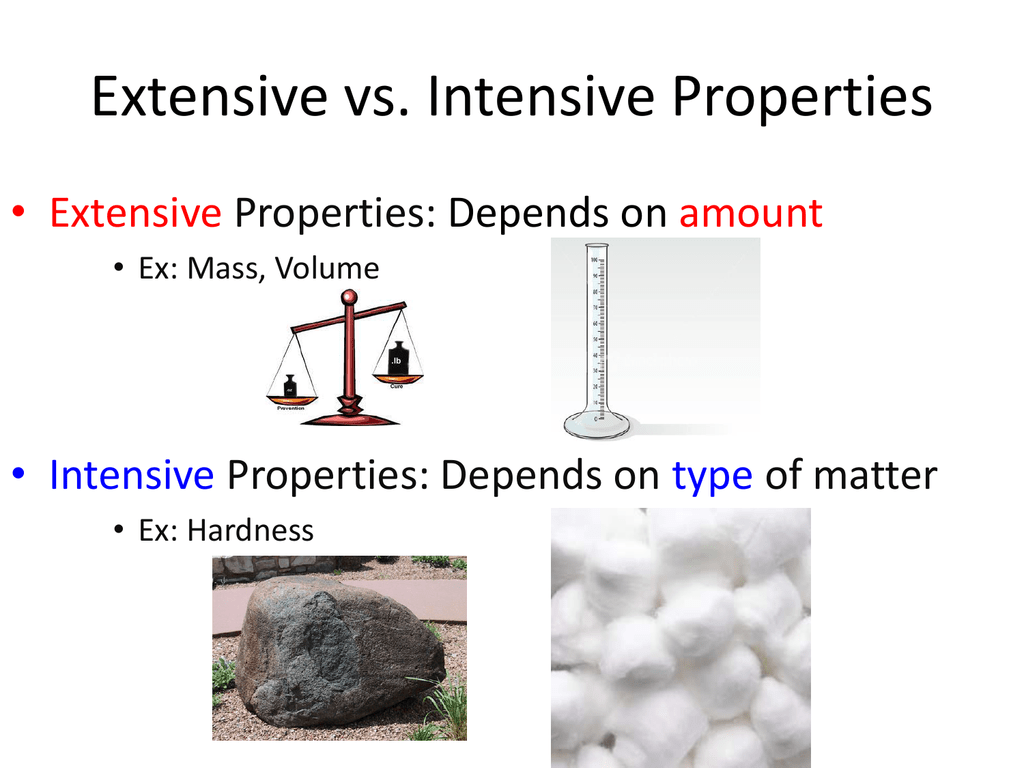
Differentiate Between Extensive And Intensive Properties - For example, in thermodynamics, the state of a. Extensive properties vary with the amount of the substance and include mass, weight, and volume. The distinction between intensive and extensive properties has some theoretical uses.
Extensive properties vary with the amount of the substance and include mass, weight, and volume. For example, in thermodynamics, the state of a. The distinction between intensive and extensive properties has some theoretical uses.
For example, in thermodynamics, the state of a. Extensive properties vary with the amount of the substance and include mass, weight, and volume. The distinction between intensive and extensive properties has some theoretical uses.
Difference Between Intensive and Extensive Properties Definition
The distinction between intensive and extensive properties has some theoretical uses. For example, in thermodynamics, the state of a. Extensive properties vary with the amount of the substance and include mass, weight, and volume.
Difference Between Intensive and Extensive Properties of Matter
The distinction between intensive and extensive properties has some theoretical uses. Extensive properties vary with the amount of the substance and include mass, weight, and volume. For example, in thermodynamics, the state of a.
Difference Between Intensive And Extensive Properties
The distinction between intensive and extensive properties has some theoretical uses. For example, in thermodynamics, the state of a. Extensive properties vary with the amount of the substance and include mass, weight, and volume.
What is the difference between extensive properties and intensive
For example, in thermodynamics, the state of a. The distinction between intensive and extensive properties has some theoretical uses. Extensive properties vary with the amount of the substance and include mass, weight, and volume.
Extensive vs. Intensive Properties — Overview & Examples Expii
Extensive properties vary with the amount of the substance and include mass, weight, and volume. For example, in thermodynamics, the state of a. The distinction between intensive and extensive properties has some theoretical uses.
Extensive vs Intensive Properties Difference and Comparison
Extensive properties vary with the amount of the substance and include mass, weight, and volume. The distinction between intensive and extensive properties has some theoretical uses. For example, in thermodynamics, the state of a.
Intensive/Extensive Properties Activity
The distinction between intensive and extensive properties has some theoretical uses. Extensive properties vary with the amount of the substance and include mass, weight, and volume. For example, in thermodynamics, the state of a.
Extensive vs. Intensive Properties
Extensive properties vary with the amount of the substance and include mass, weight, and volume. The distinction between intensive and extensive properties has some theoretical uses. For example, in thermodynamics, the state of a.
Intensive and Extensive Properties Worksheet
Extensive properties vary with the amount of the substance and include mass, weight, and volume. The distinction between intensive and extensive properties has some theoretical uses. For example, in thermodynamics, the state of a.
The Distinction Between Intensive And Extensive Properties Has Some Theoretical Uses.
Extensive properties vary with the amount of the substance and include mass, weight, and volume. For example, in thermodynamics, the state of a.





:max_bytes(150000):strip_icc()/intensive-vs-extensive-properties-604133-v3-5b55fb394cedfd0037117796.png)