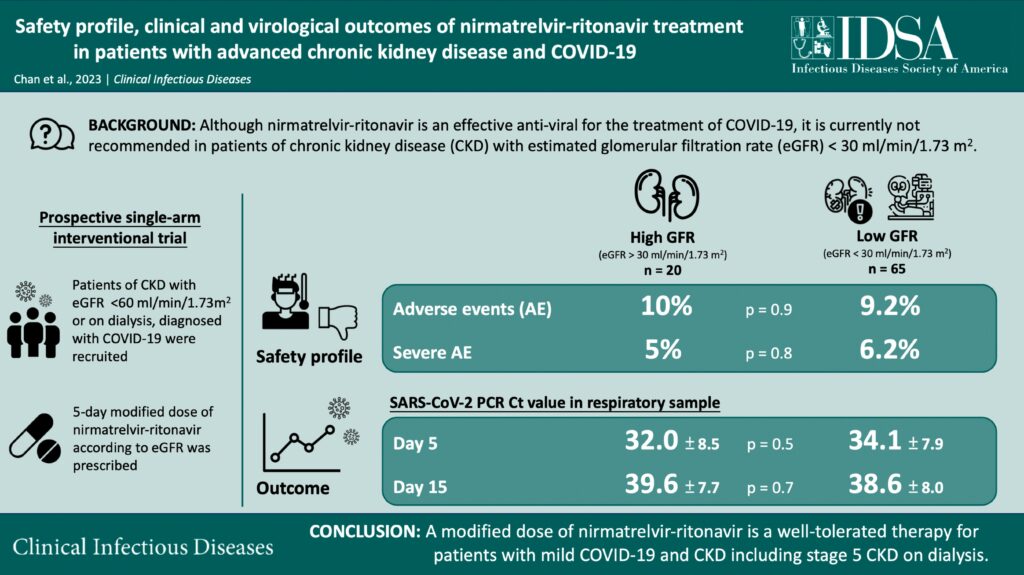
Does Paxlovid Prevent Pneumonia - Recently, several oral antiviral drugs have been reported to reduce the frequency of hospitalization, inhibit risk of pneumonia. Paxlovid is commonly prescribed in patients with mild and normal pneumonia within 5 days from the symptom onset. In one clinical trial, paxlovid. 0.4%, p < 0.0001), hospitalization (0.1% vs. 1.2%, p < 0.0001), and death rates (0.04% vs.
0.4%, p < 0.0001), hospitalization (0.1% vs. 1.2%, p < 0.0001), and death rates (0.04% vs. Recently, several oral antiviral drugs have been reported to reduce the frequency of hospitalization, inhibit risk of pneumonia. Paxlovid is commonly prescribed in patients with mild and normal pneumonia within 5 days from the symptom onset. In one clinical trial, paxlovid.
Recently, several oral antiviral drugs have been reported to reduce the frequency of hospitalization, inhibit risk of pneumonia. 0.4%, p < 0.0001), hospitalization (0.1% vs. Paxlovid is commonly prescribed in patients with mild and normal pneumonia within 5 days from the symptom onset. In one clinical trial, paxlovid. 1.2%, p < 0.0001), and death rates (0.04% vs.
COVID drug Paxlovid, which helps prevent severe symptoms, will double
0.4%, p < 0.0001), hospitalization (0.1% vs. 1.2%, p < 0.0001), and death rates (0.04% vs. Recently, several oral antiviral drugs have been reported to reduce the frequency of hospitalization, inhibit risk of pneumonia. Paxlovid is commonly prescribed in patients with mild and normal pneumonia within 5 days from the symptom onset. In one clinical trial, paxlovid.
F.D.A. Advisers Endorse Paxlovid’s Benefits as a Covid Treatment The
1.2%, p < 0.0001), and death rates (0.04% vs. In one clinical trial, paxlovid. 0.4%, p < 0.0001), hospitalization (0.1% vs. Paxlovid is commonly prescribed in patients with mild and normal pneumonia within 5 days from the symptom onset. Recently, several oral antiviral drugs have been reported to reduce the frequency of hospitalization, inhibit risk of pneumonia.
Paxlovid does not prevent 'long COVID,' UCSF study shows
Recently, several oral antiviral drugs have been reported to reduce the frequency of hospitalization, inhibit risk of pneumonia. 0.4%, p < 0.0001), hospitalization (0.1% vs. In one clinical trial, paxlovid. Paxlovid is commonly prescribed in patients with mild and normal pneumonia within 5 days from the symptom onset. 1.2%, p < 0.0001), and death rates (0.04% vs.
Paxlovid May Reduce Risk of Long Covid in Eligible Patients, Study
Recently, several oral antiviral drugs have been reported to reduce the frequency of hospitalization, inhibit risk of pneumonia. 0.4%, p < 0.0001), hospitalization (0.1% vs. 1.2%, p < 0.0001), and death rates (0.04% vs. In one clinical trial, paxlovid. Paxlovid is commonly prescribed in patients with mild and normal pneumonia within 5 days from the symptom onset.
Ask a health expert ‘Should I take Paxlovid if I have COVID?’ Fox News
Paxlovid is commonly prescribed in patients with mild and normal pneumonia within 5 days from the symptom onset. 1.2%, p < 0.0001), and death rates (0.04% vs. In one clinical trial, paxlovid. 0.4%, p < 0.0001), hospitalization (0.1% vs. Recently, several oral antiviral drugs have been reported to reduce the frequency of hospitalization, inhibit risk of pneumonia.
Paxlovid Rebound What to Watch For and Whether You Should Worry The
1.2%, p < 0.0001), and death rates (0.04% vs. 0.4%, p < 0.0001), hospitalization (0.1% vs. Recently, several oral antiviral drugs have been reported to reduce the frequency of hospitalization, inhibit risk of pneumonia. Paxlovid is commonly prescribed in patients with mild and normal pneumonia within 5 days from the symptom onset. In one clinical trial, paxlovid.
Should I take Paxlovid? What to know about the covid antiviral. The
Paxlovid is commonly prescribed in patients with mild and normal pneumonia within 5 days from the symptom onset. 0.4%, p < 0.0001), hospitalization (0.1% vs. 1.2%, p < 0.0001), and death rates (0.04% vs. Recently, several oral antiviral drugs have been reported to reduce the frequency of hospitalization, inhibit risk of pneumonia. In one clinical trial, paxlovid.
CUHKPWH research confirms Paxlovid can benefit patients with severe
In one clinical trial, paxlovid. 1.2%, p < 0.0001), and death rates (0.04% vs. Recently, several oral antiviral drugs have been reported to reduce the frequency of hospitalization, inhibit risk of pneumonia. 0.4%, p < 0.0001), hospitalization (0.1% vs. Paxlovid is commonly prescribed in patients with mild and normal pneumonia within 5 days from the symptom onset.
Should You Take Paxlovid? What to Know About the Covid Treatment The
In one clinical trial, paxlovid. Paxlovid is commonly prescribed in patients with mild and normal pneumonia within 5 days from the symptom onset. 0.4%, p < 0.0001), hospitalization (0.1% vs. 1.2%, p < 0.0001), and death rates (0.04% vs. Recently, several oral antiviral drugs have been reported to reduce the frequency of hospitalization, inhibit risk of pneumonia.
Paxlovid for COVID Side effects, drug interactions, what to know
1.2%, p < 0.0001), and death rates (0.04% vs. Recently, several oral antiviral drugs have been reported to reduce the frequency of hospitalization, inhibit risk of pneumonia. Paxlovid is commonly prescribed in patients with mild and normal pneumonia within 5 days from the symptom onset. 0.4%, p < 0.0001), hospitalization (0.1% vs. In one clinical trial, paxlovid.
0.4%, P < 0.0001), Hospitalization (0.1% Vs.
Recently, several oral antiviral drugs have been reported to reduce the frequency of hospitalization, inhibit risk of pneumonia. Paxlovid is commonly prescribed in patients with mild and normal pneumonia within 5 days from the symptom onset. In one clinical trial, paxlovid. 1.2%, p < 0.0001), and death rates (0.04% vs.